Talk:Greenhouse gas/Archive 4
| This is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 | Archive 2 | Archive 3 | Archive 4 | Archive 5 | Archive 6 |
Inclusion of table of CO2 emissions from various fossil fuels
As it is, the table proposed for inclusion is in US units. Would be nice to have it in SI units also.
Kg/MJ Natural gas 0,05030 Liquefied petroleum gas 0,05976 Aviation gasoline 0,06578 Automobile gasoline 0,06707 Kerosene 0,06836 Tires/tire derived fuel 0,08126 Wood and wood waste 0,08383 Coal (bituminous) 0,08813 Coal (subbituminous) 0,09157 Coal (lignite) 0,09243 Petroleum coke 0,09673 Coal (anthracite) 0,09759
I don't know how to do that in a nice way. Gearløs (talk) 20:07, 14 January 2009 (UTC)
- I'm far from confident about the accuracy of those figures, to say that all bituminous coal would givee off that amount for example...I'm also not sure this article needs those figures...maybe in a 'human sources of CO2' article if there is one, or...maybe in the actual CO2 article, think it's a bit specific for this one....are we going to do that for methane and the rest as well, including natural sources? Can't see that happening....92.6.233.226 (talk) 17:48, 15 January 2009 (UTC)
- I just noted that there is actually a table of fuels and their CO2 emissions in the article as of now. It is written in terms of pounds per Btu. Very anglocentric. I'd like that to be more international either by rewording it to kg per MJ or by adding a column with kg per MJ. Accuracy of figures is important of course, but isn't it clear that any such figures would only be approximate anyway? Yes, my figures above have too many decimals.
Gearløs (talk) 09:47, 7 April 2009 (UTC)
Absolutely ridiculous sentence in intro
"Greenhouse gases, mainly water vapor, are essential to helping determine the temperature of the Earth; without them this planet would likely be so cold as to be uninhabitable."
Without the greenhouse gas carbon dioxide there would be no life as we know it. Essentially all carbon in all living beings comes from carbon dioxide in the atmosphere. Without carbon dioxide the Earth would be lifeless no matter what the temperature. blackcloak (talk) 08:27, 25 February 2009 (UTC)
Table needed
This section badly needs to be transformed into a table. I'm terrible with and tables and even if I could figure it out I most definitely do not have the time. If anyone would be willing to do that it would be great. Bsimmons666 (talk) 21:41, 29 March 2009 (UTC)
Picture needs to be updated.
This image should replace the picture entitled "Total Greenhouse Gas Emissions."
[1] --71.249.115.165 (talk) 23:16, 22 April 2009 (UTC)
Burning Fossil Fuels can produce two times as much water as CO2
The statement "human activity does not directly affect water vapor concentrations except at local scales, such as near irrigated fields" seems misleading. Water vapor is produced when fossil fuels are burned. For a pure alkane hydrocarbon the amount of water to cardon dioxide is 1 H2O per 1 CO2 + 1 H2O.
CH4+ 2O2 = CO2 + 2H2O
I know the over all percentage is less but only becuase the amount of water in the atmosphere is greater. Maybe it should read "human activity does not significantly affect water vapor concentrations except at local scales, such as near irrigated fields". Please someone respond to this so I know where the rest of you stand. Thank you —Preceding unsigned comment added by 65.67.110.35 (talk) 22:07, 27 April 2009 (UTC)
- Sure, that's more accurate for the reasons you state: "directly" -> "significantly." I'll change it. By the way if you register for an account you'll be able to edit the article once the account has been around a while. Short Brigade Harvester Boris (talk) 02:54, 28 April 2009 (UTC)
- No, it isn't quite like that. This is because water vapor is in equilibrium with liquid (and solid) water, while liquid or solid carbon dioxide do not occur naturally on Earth to maintain an equilibrium. 69.140.12.180 (talk) 00:35, 22 May 2009 (UTC)Nightvid
- Since the water released by burning fossil fuels remains in the atmosphere for only about 10 days, it is usually ignored. However, because fuels are continuously burned, there is a new higher equilibrium. The increase is approximately equal to 10 times what is produced each day. However, this is still considered to be insignificant. Q Science (talk) 06:37, 22 May 2009 (UTC)
- This not the whole story. There is a large reservoir of "liquid" CO2 available for entering the atmosphere in the oceans. It's not liquid in the sense of concentrated CO2, which exists under pressure and is used industrially. The liquid form is as dissolved CO2 in water. When pure rain forms in the clouds, the water is loaded with CO2, presumably to the maximum extent allowed by its soluability (temperature probably plays a part as well). Of course this is "pure" water with a fairly neutral pH. And effectively the CO2 payload in each raindrop is from the atmosphere at the altitude of the cloud in which the raindrop formed. When the raindrop meets the ocean, the CO2 becomes integrated into the salt water, and enters an environment that has a very different pH. These conditions form a different environment for soluability of CO2 in the sea water chemical mix. Because sea water is constantly in contact at the surface with the atmosphere, equilibrium conditions can be assumed to be established. The arrival of a raindrop alters this equilibrium. The question I have not resolved is whether or not a portion of the CO2 that has entered the surface of the sea water from the raindrop is ejected into the atmosphere (which I think is what happens) or the conditions of the surface layer of the sea water are altered by the arrival of the raindrop in such a way that more CO2 must then enter the sea water from the atmosphere in order to re-establish equilibrium conditions. Whatever happens, rain is a mass transport system, moving CO2 from high in the atmosphere to the Earth's surface. blackcloak (talk) 19:42, 24 December 2009 (UTC)
Incorrect use of the concept of feedback in section 4. Role of Water Vapour
The following statement is incorrect with regard to the definitions of positive and negative feedback: In climate matters, when a warming trend results in effects that induce further warming, the process is referred to as a "positive feedback"; when the effects induce cooling, the process is referred to as a "negative feedback"
This statement implies that temperature is influenced in the same direction as the "sign" of the feedback. This is wrong because you could have a cooling positive feedback system and negative feedback tends to stabilise a system's output. It would be best to simply remove this incorrect use of feedback from the article since it is likely only to confuse.
You can look up the proper definitions but off the top of my head, this is how I understood it from my engineering mathematics at university: Feedback on a system is where the output of that system contributes to the input of the system as a function of the output. If the feedback increases the output, which in turn increases the feedback, then this is positive feedback and leads to an unstable system. Negative feedback on the other hand reduces the input signal on the system, so the higher the output, the stronger the negative feedback becomes, negativing the initial source input, and tending to a stable output (depending on the system function of course). —Preceding unsigned comment added by Karlburgdorf (talk • contribs) 11:28, 30 May 2009 (UTC)
- The statement is correct as written. Karlburgdorf's definition also is correct. This statement implies that temperature is influenced in the same direction as the "sign" of the feedback. - no it doesn't, though it could be written more clearly. Short Brigade Harvester Boris (talk) 15:05, 30 May 2009 (UTC)
What about the history? ... or Venus? ... or Hoyle? etc.
This article's primary problem, at this point, is that there's no clear delineation between it and greenhouse effect (I actually question why greenhouse gas needs to exist as separate from greenhouse effect, since Greenhouse_effect#Greenhouse_gases is already a sub-section which appears to contain all of the unique information from this article).
Both articles, however, focus far too much on Earth. In 1955, Hoyle introduced the idea of a runaway greenhouse effect. In the mid-to-early 1960s an explosion of work in this field resulted from the Mariner 2 flyby of Venus between 1963 and 1964 (see refs from http://books.nap.edu/openbook.php?record_id=1790&page=235). It is from this primarily extra-terrestrial focus that modern greenhouse theory was born. Why, then, does the word "Venus" appear once in this article? Why do neither this nor greenhouse effect talk about the development and history of this theory through the 1950s, 1960s or 1970s?! These are essentially, just spin-off articles of global warming that exist to reiterate what that article contains.
I'm not taking a political stand, here. Nor am I saying that I think the content of this article is wrong or inaccurate. I'm just saying that the title of the article and the content are not related in the way that a typical reader of Wikipedia would expect. -Miskaton (talk) 22:17, 5 June 2009 (UTC)
- Why, then, does the word "Venus" appear once in this article? Why do neither this nor greenhouse effect talk about the development and history of this theory through the 1950s, 1960s or 1970s?! - because you haven't bothered to write it in William M. Connolley (talk) 22:42, 5 June 2009 (UTC)
- That's an easy answer, of course, but regardless of my participation, here, I think these were valid questions that anyone working on this article should be asking. If I were to contribute to this page, it would likely be to add a merge suggestion tag to it. -Miskaton (talk) 04:25, 6 June 2009 (UTC)
Overstatement
I adjusted "Greenhouse gases, mainly water vapor, are essential to helping determine the temperature of the Earth; without them this planet would likely be so cold as to be uninhabitable" in the lead, as it seemed to be untestable overstatement. --John (talk) 03:03, 8 June 2009 (UTC)
- It's hard to imagine an inhabitable Earth with no liquid water, but I don't think the statement is necessary. Short Brigade Harvester Boris (talk) 03:14, 8 June 2009 (UTC)
Article fails to explain the basic mechanism (if any) of greenhouse gas operation
The article states that greenhouse gases absorb and re-emit radiation. Doesn't that mean no net change in the radiation ? That is, after the absorption/re-emission, the radiation is still there. The greenhouse gases had no effect.
It is possible that the re-emission occurs at a frequency which has different properties than the incoming (absorbed) frequency. If so, the article fails to explain this.
It is also possible that actual "absorption" is not occurring. It could be that "reflection" is occurring. For example, the ionosphere reflects radio waves (another type of radiation). Thus, under the reflection scenario, radiation strikes the surface of the earth, is reflected there, and then re-reflected by the greenhouse gases back to the surface.
Further, numerous factors remain unexplained. Suppose that the greenhouse gases absorb at frequency F. That would mean that, in sunlight reaching the surface of the earth, frequency F would be diminished or eliminated, compared to frequency F detected by a satellite in space. Thus, the energy in frequency F is absorbed partially or completely by the atmosphere, en route to the surface. The atmosphere gets heated instead of the surface. Why is that a problem ?
The following is a major problem.
The article fails to address a basic issue which arises when the system is modelled from a heat-transfer perspective. The diameter of the earth is 8,000 miles. The thickness of the atmosphere is (pick a number) 20 / 40 / 60, but no more than 100 miles. If 80 miles thick (which is not likely) then the thickness of the atmosphere is 80/8,000, or 8/800, or 1/100, or one percent of the diameter.
By comparison, if the earth is viewed as a softball 5 inches in diameter, then the atmosphere is 5 x 0.01, or 0.05 inches thick. That is the thickness of 10 sheets of paper.
This softball, with the "paper" coating, is placed into space, and illuminated by the sun. The softball absorbs some heat, and radiates some heat, and reaches a steady-state temperature.
How does changing the properties of the (very thin) "paper" coating (by adding greenhouse gases) affect the temperature of the softball ?
I have a dozen more points. The absence of a proposed mechanism by which greenhouse gases supposedly work is a serious problem. It suggests to me bias, in the sense that it is assumed that "greenhouse gases" are causing warming of the climate, with little proof.
END —Preceding unsigned comment added by 208.103.35.248 (talk) 16:38, 30 June 2009 (UTC)
- Most, if not all, of these questions are answered in the Greenhouse effect article, which is cited as "main article" in the Greenhouse gases in Earth's atmosphere section of this one. Suggestions for improvement in the explanation of the greenhouse effect should be made on that article's talk page. Agathman (talk) 18:54, 30 June 2009 (UTC)
Can someone fix this?
The first paragraph says "without them, Earth's surface would be on average about 33°C (59°F) colder than at present."
But 33 C is close to 90F and 59F is about 15C.
Which is right? Gwilson (talk) 00:35, 23 July 2009 (UTC)
- These are delta degree's not absolute degrees. An increase/decrease of 33°C is the same as an increase/decrease of 59°F. (0°C is not the same as 0°F) --Kim D. Petersen (talk) 00:52, 23 July 2009 (UTC)
"human activity does not significantly affect water vapor concentrations except at local scales"
In the article, under water vapour, it is stated without reference that "human activity does not significantly affect water vapor concentrations except at local scales". I am unclear how this can be true, given that in all cases except coal, the burning of hydrocarbons by defintion also release water. From the greatest fraction - burning methane releases two water molecules for every one molecule of carbon dioxide - to lower fractions for higher wieght oils - at c50 the fraction is 26 molecules of water and 50 molecules of carbon dioxide. Can someone provide a reference for the above statement, or have I missed something here? Keeper50 (talk) 19:03, 18 August 2009 (UTC)keeper50
- In addition, water is used to cool power plants, and that also releases a lot of water vapor. However, the lifetime of water vapor in the atmosphere is only a few days. Therefore, the concentration will not increase over time. Thus, while it is true that human actions increase the water vapor, the amount truly is insignificant "except at local scales". In the same way, the amount of CO2 that humans add to the atmosphere in 10 days is also totally insignificant. The problem with CO2 is that the increase will remain in the atmosphere for 30 to 100 years and, over time, will increase to a significant amount. Q Science (talk) 19:17, 18 August 2009 (UTC)
- (EC) The article doesn't say there is no effect from human activity, only that it isn't significant. Back of the envelope calculation: Global oil and gas usage is about 100 million bbl/day which is roughly 12 million tonnes. Assuming two atoms of H for each atom of C yields 1.7 million tonnes of H, corresponding to 15 million tonnes of H2O. Global daily evapotranspiration per unit area is around 2.8 mm/day, which works out to 1.4 x 1012 tonnes of H2O. So on a global average, fossil fuel combustion produces just about one millionth of the amount of water that enters the atmosphere through evapotranspiration. somebody check my math Short Brigade Harvester Boris (talk) 19:24, 18 August 2009 (UTC)
- While I have not checked your starting point numbers, it looks to me like you made an error when dividing. 15*10^6 tonnes of H2O entering the atmosphere per day from fossil fuel consumption by man divided by 1.4*10^12 tonnes of H20 entering the atmosphere per day by natural (presumably solar energy driven) evaporation is about 1/100,000. Then, if we use the presumed lifetime of 10 days residency in the atmosphere, we would estimate that 1/10,000 or .01% of the water vapor in the atmosphere at any given time is due to human fossil fuel consumption. This means that, roughly, one millionth of the weight of the atmosphere at any given time is additional water vapor due to human fossil fuel consumption. Assuming that the greenhouse effect were entirely due to water vapor, and assuming a linear dependence on concentration, 300degK/10,000 implies that the temperature rise due to additional water vapor entering the atmosphere due to human consumption of fossil fuel can not exceed 1/30 degree C. blackcloak (talk) 06:39, 7 December 2009 (UTC)
- Since you seem to have an ability to work with these back of the envelope calculations, care to try your hand at another one? Estimate the amount (weight) of CO2 flushed from the atmosphere daily by rain. Estimate the proportion of this "removed" CO2 that re-enters the atmosphere immediately. blackcloak (talk) 06:39, 7 December 2009 (UTC)
Reference Requested
I am trying to obtain a reference paper for the following unreferenced statement: "Both CO2 and CH4 vary between glacial and interglacial phases, and concentrations of these gases correlate strongly with temperature". Can anyone provide a paper for me to use? Keeper50 (talk) 19:03, 18 August 2009 (UTC)keeper50
- You can plot the actual vostok data yourself. As for the correlation, there are references to support and oppose that statement. A discussion of the correlation problem is provide by Dr Hansen (2007). Q Science (talk) 05:44, 20 August 2009 (UTC)
Greenhouse gases (GHGs) acronym
Shouldn't there be a (GHGs) after the first instance of 'Greenhouse gases'? Later on in the article GHG and GHGs are used quite often... Sim0n (talk) 08:32, 27 September 2009 (UTC)
Incorrect sentence (Two papers from 1997/1999 argue against a future paper in 2002?).
One study using evidence from stomata of fossilized leaves suggests greater variability, with carbon dioxide levels above 300 ppm during the period seven to ten thousand years ago[33], though others have argued that these findings more likely reflect calibration or contamination problems rather than actual CO2 variability.[34][35]
The first part of this sentence refers to a 2002 paper[33], the second part refers to two papers from 1997 and 1999[34][35]. I highly doubt that these two papers (1997&1999) already knew the findings of the 2002 paper. So either the wording of the sentence is wrong (they did not argue against the findings, as these findings did not exist in 1999 & 1997), or something else is incorrect.
/this is my first 'edit' on wikipedia, so be kind ;) —Preceding unsigned comment added by Jbinken (talk • contribs) 06:28, 10 October 2009 (UTC)
References 53 and 54 should be removed
These two references have problems so serious that they should be removed, and, at the very least, replaced with links to current primary source documents. References to abstracts should be considered useless within wikipedia because they do not allow everyone direct access to the underlying material. There is one broken link. Does wikipedia have a policy regarding links to material that require a payment for access? It becomes too easy to misuse references when the material can not be accessed by everyone. blackcloak (talk) 08:27, 25 October 2009 (UTC)
- Hard to find and pay-wall works are entirely allowed. You could visit a university library if you don't want to pay the online fee to read scientific papers. Wikipedia is an encyclopedia based on all reliable sources, not just those sources that can be found on the internet. Dragons flight (talk) 15:04, 25 October 2009 (UTC)
- If a link is provided, it should be to material that justifies the content of the article. While a reference to printed matter is entirely appropriate (no link) as in a printed publication, a link to an abstract that does not in itself contain the information required substantiate the article's content is not appropriate. I regard it as a childish prank because all it does is waste the reader's time and become a source of frustration. I don't think that is what wikipedia should be tolerating. As a suggestion, I would propose that all such links be explicitly designated with a notation such as (pay). Basically, I think you missed the point of my comment, as nothing you said goes beyond the obvious. blackcloak (talk) 05:19, 29 October 2009 (UTC)
- The link is a reference to the source of the info. If the link happens to include the actual info, that is a bonus. A link to an abstract is fine. William M. Connolley (talk) 09:16, 29 October 2009 (UTC)
- If this is to be wikipedia's position on links in references, then I shall adopt a personal policy of never clicking on a link in a reference. This will serve to reduce my level of frustration with (and unfortunately, trust in, and reliance upon) this resource. 67.87.73.86 (talk) 22:27, 29 October 2009 (UTC)
Will someone please quote the actual text (place in this section, just below my entry) of the articles (ref 53 and 54) that supports the statement on "effective lifetime." And if the authors define the term "effective lifetime," what is their definition? Do the authors provide any additional estimates of "lifetime" such as ones more in keeping with way physicists use the term, in particular their estimate of the half life of CO2 in the atmosphere? blackcloak (talk) 09:52, 5 November 2009 (UTC)
First two sentences still have a problem
The first sentence tells us what greenhouse gases are. Within that sentence there is no description of anything that we might call a process. The second sentence starts with "This process ...", the antecedent of "this" being where? Well, it's not there. How can editors be so thoughtless? Doesn't anyone pay attention to detail anymore? What is interesting is that, had a process been defined within the first sentence, the use of the word "cause" might be defensible. As they are, the two sentences start with a logical inconsistency that leads to a rational, though wrong, generalization. There is a word for what is going on here. It's called sophistry. blackcloak (talk) 20:04, 1 December 2009 (UTC)
- WP:SOFIXIT. -Atmoz (talk) 20:13, 1 December 2009 (UTC)
- Be my guest. If not, others need the practice. I need neither the practice nor the aggravation. Teach a man to fish, and perhaps he'll teach another man to fish. blackcloak (talk) 07:00, 2 December 2009 (UTC)
Methane increase 149%
In the table of increase in GHG from pre-industrial times, methane increase from 700 ppb to 1745 ppb is not 67% increase but 149% increase. Stamnon (talk) 10:50, 15 December 2009 (UTC)15 December 2009Stamnon (talk) 10:50, 15 December 2009 (UTC)
Article probation
Please note that, by a decision of the Wikipedia community, this article and others relating to climate change (broadly construed) has been placed under article probation. Editors making disruptive edits may be blocked temporarily from editing the encyclopedia, or subject to other administrative remedies, according to standards that may be higher than elsewhere on Wikipedia. Please see Wikipedia:General sanctions/Climate change probation for full information and to review the decision. -- ChrisO (talk) 19:37, 2 January 2010 (UTC)
Portrayal of anthropogenic global warming theory as fact
I'm not familiar with how this whole thing works, so apologies in advance if I do something stupid.
Wikipedia articles in general have been an excellent resource, but I'm a little concerned with an apparent bias of the greenhouse gas one. The line that I find most disturbing is:
"Human activities since the start of the industrial era around 1750 have increased the levels of greenhouse gases in the atmosphere."
This seems no more than a mere parroting of what we have been hearing on TV, and certainly not worthy of being encyclopedic content. Given that no evidence exists to support such a direct statement, I made a few edits to present the information in a more neutral manner (along with minor corrections) which were rejected. May I ask why? —Preceding unsigned comment added by 96.236.8.229 (talk) 04:56, 14 January 2010 (UTC)
- You're right to point out that the statement as it stood in the article was unsupported, less correct to claim that there is no scientific support for the statement. I've added a link to this FAQ at the US government's Carbon Dioxide Information Analysis Center. --TS 22:58, 23 February 2010 (UTC)
- Good reference. Go to link in the answer to the second question. Then read footnote 4. You'll see that this US government source uses a totally different way of describing "atmospheric lifetime" from the way this article defines the term. Not only does it describe the use of a short impulse function, it describes decay as a physicist would, with an exponential and a one over e decay time. Obviously there are individuals who have some understanding of the subject, unlike the editors who wrote the sections of this article that refer to atmospheric lifetime. blackcloak (talk) 07:17, 24 February 2010 (UTC)
Methane
In a recent edit, WMC removed an EPA reference that stated
- Methane is over 20 times more effective in trapping heat in the atmosphere than carbon dioxide (CO2) over a 100-year period
and replaced the text with
- methane is about eighty times stronger greenhouse gas than carbon dioxide
with the following explanatory note
- twenty is over 100 y (GWP) not instantaneous. eight is obviously wrong - I think 80 is most likely
The methane article says that methane has a
- global warming potential of 72 (calculated over a period of 20 years) or 25 (for a time period of 100 years).
which does not appear to be supported by the provided reference (IPCC).
At any rate, please restore the EPA reference and value, or provide an appropriate reference for the "80 times stronger". Either way, the methane page also needs to be fixed. Q Science (talk) 08:07, 27 April 2010 (UTC)
- Yes. The point is that we're talking about the direct radiative forcing in this place, not the time-integrated GWP. Because methane decays faster than CO2, its instantaneous radiative forcing (per molecule) is higher relative to CO2 than its long-term GWP William M. Connolley (talk) 08:39, 27 April 2010 (UTC)
- No I haven't. I'm guessing. It is consistent with the 72 over 20 years though - whatever the number is, it should be a little higher than that William M. Connolley (talk) 09:26, 28 April 2010 (UTC)
- You might like to look at WP:OR and WP:SYNTH. Perhaps we can come up with a form of words which is directly supported by the source. Stephen B Streater (talk) 10:22, 28 April 2010 (UTC)
Rewrite of section on emissions
I've rewritten the section on GHG emissions. I felt that the previous revision did not offer a suitably broad coverage of the topic:
- It concentrated on particular time periods for measuring emissions,
- The global coverage was inadequate
- There wasn't sufficient information presented on cumulative emissions
- There was a bias towards discussing emissions in China and the US.
On point 1, the previous revision was rather arbitrary in its coverage of time periods. Things were mixed together and I felt that they would be better presented in separate categories. The new revision has information on emissions over the 1970-2004 time period, as well as the 1990-present. I've kept some info from the previous revision on measurements since 2000.
On point 2, it doesn't make sense to concentrate only on China, the US, and the UK. The subject needs to be explained from a global perspective. I've attempted to do this in a short a space as possible. I've put in a link to the article on Kyoto Protocol and government action. This article more comprehensively describes emissions and climate policies in a wide number of countries.
Point 3: I've added info on cumulative emissions from the IEA.
Point 4: This is related to point 2. Emissions in these countries are important, but it is biased only to discuss emissions within the context of comparing emissions between these two countries. This is a rather arbitrary thing to do. The US and China are important emitters, but that does not mean that this article should concentrate so much on their actions. A global perspective should look at the issue in broader terms, e.g., Annex I v. non-Annex I, developed versus developing. For this article, these kind of groupings are more appropriate since they include a far larger number of countries. The Kyoto article I referred to previously offers a broader description of emissions in various countries. Enescot (talk) 12:56, 22 May 2010 (UTC)
Units
The table "Relative CO2 emission from various fuels" should be in SI-units. —Preceding unsigned comment added by ArnaudContet (talk • contribs) 16:00, 24 May 2010 (UTC)
Add Category:Emissions reduction ? 99.37.85.55 (talk) 18:07, 25 May 2010 (UTC)
Inconsistent radiative forcing figures.
Radiative forcing figures for CO2 are inconsistent across article. They were probably counted with:
- P = 3.35*ln(1 + 1.2*C + 0.005*C^2 + 0.0000014*C^3)
- where C is CO2 in parts per million and P is radiative forcing
...but something wrong happened while updated. If I'm right this numbers should be:
For 280->387 ppm: 1.79 W/m^2 (instead of 1.46 in section "Natural and anthropogenic", "current level" updated but radiative forcing not?)
For 280->365 ppm: 1.46 W/m^2 (that's right - section "Greenhouse gas emissions")
For 280->383 ppm: 1.73 W/m^2 (instead of 1.53 in section "Greenhouse gas emissions", typo? "5" instead of "7"?) —Preceding unsigned comment added by 89.246.67.228 (talk) 13:33, 31 May 2010 (UTC)
- This is what I found (Please expand and correct as appropriate.)
CO2 change Article Reference Computed Reference 278 -> 365 1.46 1.46 1.46 [IPCC - ref 41 in main document] 280 -> 384.8 none 1.66 1.76 [ref 42 in main document] 280 -> 383 1.53 none 1.73 none 280 -> 387 1.46 none 1.79 none
- Since computing values instead of using those in the references is considered WP:OR, I don't think it is correct to change the values, but it is a good idea to add a column with the computed values and to delete all values that do not have references. Q Science (talk) 23:25, 31 May 2010 (UTC)
Statistics by industry
It would be very illuminating to have statistics showing what percentage of each major greenhouse gas come from each industry. For example, a 2006 U.N. report claims that more global warming is caused by livestock than transportation: [2] -- Beland (talk) 15:18, 5 July 2010 (UTC)
fossil fuels
The statement which makes fossil fuels the villain is not adequately referenced. The current statement says: "The burning of fossil fuels since the beginning of the industrial revolution has substantially increased the levels of carbon dioxide in the atmosphere." Reference [5] is to a Q & A of the Carbon Dioxide Information Analysis Center (CDIAC). CDIAC only estimates carbon dioxide from fossil fuels and from no other source. The reference which CDIAC gives for their conclusion is an article by Richard A. Houghton on Land Use Changes. Land Use Changes do not prove anything about fossil fuel.
The alleged need for all carbon dioxide control and greenhouse gas regulation is the control of fossil fuels. For this reason, the allegation that fossil fuel is the culprit needs to have overwhelming understandable scientific references. It does not have that. Obviously, there is a reason for such omissions.
The CDIAC conclusion appears to be nothing more than an assumption since its land use reference is not evidence of its truth. It is not supported by peer-reviewed scientific studies of the many sources of carbon dioxide emissions. —Preceding unsigned comment added by 24.27.16.34 (talk) 19:53, 27 July 2010 (UTC)
- See [3] read up to and including the paragraph which starts "What about the land biosphere?" You may need to read it a few times to absorb it, I did (no pun with "absorb" here) ..in essence they can measure the net effect of both deforestation (which would increase CO2) and absorption of CO2 in the biosphere...basically by doing some "Accounting" with + and - for CO2 among oceans, atmosphere, and biosphere. This accounting shows that the extra CO2 in the atmosphere did not come from the biosphere, which actually lost 15±9 PgC net. This loss is a combination of the loss from deforestation of 24±12 PgC, and additional land uptake of 39±18 PgC in response to elevated CO2 and climate changes...but in net 15 PgC give or take, was added, So in net, CO2 at higher levels in the atmosphere did not come from the biosphere.(by comparison, fossil fuel burning was 117±5 PgC..these numbers are not annual but for the period 1980-1999) In any case CO2 at higher levels in the atmosphere did not come from the biosphere..or did it come from the oceans losing CO2 since oceans gained CO2..[a little more analysis can then match fossil fuel burning minus extra taken up by oceans and biosphere with the level of increase we measure in the atmosphere..but that's a step beyond your question, which was merely, can we rule out that the biosphere including deforestation, is the cause of the huge [4] increase in CO2, and the answer is, yes, we can] --Harel (talk) 01:23, 19 August 2010 (UTC)
Opening sentence too vague
I'm not sure about the opening sentence of the article, "Greenhouse gases are gases in an atmosphere that absorb and emit radiation within the thermal infrared range." There is more to distinguish a greenhouse gas than that it simply "absorb[s] and emit[s] radiation within the thermal infrared range". As the diagram in the lead makes clear (as do any number of textbooks), a greenhouse gas is one that is relatively transparent to high temperature (high frequency) IR, but relatively opaque to lower temperature (lower frequency) IR. Then it produces the differential effect of letting the high-temperature solar radiation in, but interfering with some of it's loss back into space at the lower temperatures of the planet and its atmosphere. How to summarise this into one clear opening sentence? It has to be longer, with more information in it than the current sentence. How about, "Greenhouse gases are gases in an atmosphere that selectively absorb and re-emit radiation more readily within the infrared region that is equivalent to the planet's surface temperature than they do for higher temperatures."? With suitable links to other articles about infrared and maybe black-body temperature, this would be clearer, IMHO. --Nigelj (talk) 12:05, 23 August 2010 (UTC)
- The opening summary is fine. If you want more details, please place them in the appropriate section. Also, "re-emit" is a common error. Even if no energy is absorbed at a given frequency, a greenhouse gas will still emit energy because of its current temperature. If fact, both water vapor and CO2 tend to emit more energy than they absorb because they are also heated by direct contact with the surface. Q Science (talk) 18:49, 23 August 2010 (UTC)
- Actually, it mentions 5 real gases by name. (The main article mentions several more.) It also says "thermal infrared range" which I interpret to mean "not" the high frequency range. However, that is not clarified in the body of the article, and I agree with you that it should be. Q Science (talk) 23:06, 23 August 2010 (UTC)
"A .01% increase in the total volume of CO2 in the atmosphere"
I don't see a need for this statement considering that it repeats a point just made in ppm ("the concentration of carbon dioxide has increased by about 36% to 380 ppmv, or 100 ppmv over modern pre-industrial levels") but in a more confusing way, so I removed it from the article. I see that 68.109.66.87 (talk · contribs) has just reverted me, saying, "provides figure of comparison, e.g. water vapor is cited as 2% of the atmosphere, as opposed to 20,000 ppmv." Well I can see that as an argument for putting "(0.038%)" after the mention of 380 ppmv, but this does seem a strange full sentence to add after the mention of the 100 ppmv change. The reason we sometimes use % and sometimes ppm is because of the size of the fraction. ppm values in the tens of thousands and percentages in the thousandths of a percent are unnecessary and wrong, as would be geographic distances in inches or insect dimensions in nautical miles. I am not prepared to edit war; perhaps people would like to discuss it. --Nigelj (talk) 16:52, 25 October 2010 (UTC)
- You were correct to delete it.
- This amounts to a .01% increase in the total volume of CO2 in the atmosphere since 1750.
- is very confusing. A 36% increase produces a 36% increase in volume, not a 0.01% increase, which is how I read the statement. I can see what the person is trying to say, but that sentence is very confusing. I suggest removing the sentence and, if the person adding this wants to, adding a note that explains the idea in a way that is easier to understand. Q Science (talk) 18:10, 25 October 2010 (UTC)
- I agree and have removed it again. As worded, it seems to be a flat contradiction of the previous sentence. Squiddy | (squirt ink?) 19:53, 25 October 2010 (UTC)
- I agree, saying CO2 increased by 0.01% by volume is simply incorrect. If the total volume of the atmosphere were one million parts, then there are 280 parts preindustrial and 380 parts currently (true if "parts" are atoms, moles, exomoles, or atoms in the atmosphere). This is a 35.7% increase in the total volume of CO2 in the atmosphere. Again removing. --TeaDrinker (talk) 00:31, 26 October 2010 (UTC)
- I agree and have removed it again. As worded, it seems to be a flat contradiction of the previous sentence. Squiddy | (squirt ink?) 19:53, 25 October 2010 (UTC)
There is obvious need for the statement, otherwise I would not have added it. Although the relative change in carbon dioxide 'concentration' in the atmosphere is significant, the absolute change in volume is very small and noting this fact in the article provides needed perspective. There is nothing incorrect about the statement. Adding ppmv to the discussion of Water vapor, or adding it after 338 ppmv would also provide this type of unit comparison, however it isn't clear that one method is significantly better than the other, but any would be an improvement. The article is not improved by the deletion of this pertinent fact. The 'argument' is for providing comparable units among the various major greenhouse gasses, rather than single digit percentages in one case, and triple digit ppm's in another. Pick one and stick with it. Your choice. 128.200.157.22 (talk) 01:55, 26 October 2010 (UTC)
- Thanks for the discussion here. I'm still not sure you're right. If I tell you old coke has 35mL caffeine per can, and the new coke has 45mL caffeine per can, that's an increase of 10mL or 10/35 = 28.6%. So I would say, to liken it to your sentence, A 28% increase in the total volume of caffeine in a can of Coke. --TeaDrinker (talk) 02:04, 26 October 2010 (UTC)
I would liken it more to saying that Coke is in a 1000 mL bottle, and Pepsi is in a 1 liter bottle. Parts per million is simply a way of expressing very small percentages with fewer decimal places in the figure. It's not unlike the purpose of scientific notation. To convert from parts per million to percent, one simply divides the ppm number by a million to get the fractional value, and then multiplies by 100 to convert to percent. Percent and ppmv are both ways to express a fraction of the total. ppm is in relation to a million, and percent is in relation to 100. 128.200.157.22 (talk) 02:46, 26 October 2010 (UTC)
- Thanks for the illustration. I follow what you are saying and your conversion, however ppm and percent are not measures of volume, they are measures of concentration. However the increase in concentration is already mentioned in the article, which is what I think is at issue. Perhaps I am not making sense... Does this clarify it? --TeaDrinker (talk) 02:55, 26 October 2010 (UTC)
One can either express these ratios in terms of volume, or in terms of weight. The convention in this article is to use volume, hence the unit ppmv. The use of volume is precisely correct. 128.200.157.22 (talk) 03:04, 26 October 2010 (UTC)
- Yes, I agree, however both are concentrations, not total volume. For example, it is not possible to convert ppm or percent into mL (a measure of total volume) or mg (a measure of total weight) without further information. Thus I don't think calling ppm or percent a measure of total volume is correct. --TeaDrinker (talk) 03:07, 26 October 2010 (UTC)
The 'v' in ppmv indicates parts per million 'volume'. However the units could just as easily be those of mass, moles, weight, or even arbitrary. But when expressed as a ratio, the units drop out of the expression. In the case of this article, the data are expressed as partial concentrations in terms of their volume - with respect either to unit volume, or to total volume. An increase in concentration of carbon dioxide per milliona parts of atmospheric volume equates to an increase in the volume of carbon dioxide in the atmosphere. It's an obvious, non-controversial point. Increasing the volume of sugar in a 100 ml can of coke from 35 ml to 45 is an increase in the volume of sugar of 10 parts per 100 because it's an increase from 35 parts per 100 to 45 parts per 100. The change in units of concentration of sugar is 10/35, but the change in volume of sugar is 10/100. I hope you can see the point. 128.200.157.22 (talk) 04:03, 26 October 2010 (UTC)
- Thanks for the explanation. I'm afraid I have to disagree. 10mL/100mL is not an expression of volume, nor change in volume. It is an expression of concentration (or actually, change in concentration). I don't see how the two could be considered interchangeable terms, since they are mathematically quite distinct. I think such a use seems to be well outside common English terminology. --TeaDrinker (talk) 04:19, 26 October 2010 (UTC)
Clearly ml is a unit of volume, and a ratio is a ratio. Though this is not a point of contention in the discussion, it is a concept with which the "editor" evidently has little facility, and difficulty grasping - a difficulty which he also fails to recognize. It is an unfortunately situation for the Wikipedia article that such people establish themselves in positions of autonomous control over technical subject matter.
- First, the use of ppmv is not correct. The correct expression is
- A concentration of x ppm
- While it is true that the numerical values of the two expressions are equal when dealing with an ideal gas, one method is correct and the other isn't. The article currently uses both expressions.
- First, the use of ppmv is not correct. The correct expression is
- Second, nobody is saying that 0.01% is wrong. It is just that the way those two percentages are used in a single paragraph is confusing. I think that a note is the best way to include that information. Also, values less than one should always be entered with a leading zero. .01 bad, 0.01 good. Q Science (talk) 05:13, 26 October 2010 (UTC)
These observations are more typographical than conceptual in nature, and do not subvert the relevance of the deleted sentence. The reasons given for the deletion remain unfounded, and their use suggests ignorance of the fundamentals (as demonstrated in the conversation above) as well as a shameful lack of impartiality to the subject matter. 68.109.66.87 (talk) 06:05, 26 October 2010 (UTC)
- I can understand your frustration, but please be assured I don't see myself as "in [a] position of autonomous control." I think everyone here has a common goal of a clear and accurate (as reflected in reliable sources) article. My concern is the use of the term "total volume." It simply does not appear to be a change in total volume. It appears to be a change in concentration, which is already included in the article. Now whether that is expressed as a change in concentration ppm, percent, or even permil, I don't think a ratio of volumes can be called a "total volume." I certainly agree the concentration went up 100ppm (which can be expressed as 0.01%, and I am happy to discuss the merits of describing it in one or the other forms), but saying the "total volume" goes up by 100ppm (or 0.01%) does not make sense to me. Expressions equivalent to ppm are simply not volume. --TeaDrinker (talk) 06:20, 26 October 2010 (UTC)
People should definitely drop the slurs on other editors' facility, impartiality, etc, particularly when defending a sentence that (at very best) is highly misleading. Saying "This amounts to a .01% increase in the total volume of CO2 in the atmosphere since 1750" reads as an assertion that the total volume of CO2 has gone up from X to X * 1.0001, whereas the figure given in the previous sentence is an increase of around 36% (ie X * 1.36). I think what you're trying to say is that the fraction of the atmosphere which is (composed of) CO2 has gone up 0.01% (not gone up by 0.01%). Wording this so that it is clear and unambiguous is tricky (I don't like the formulation I just came up with, but can't think of a better.) I also think it's superfluous, following "the concentration of carbon dioxide has increased by about 36% to 380 ppmv, or 100 ppmv over modern pre-industrial levels." Squiddy | (squirt ink?) 09:43, 26 October 2010 (UTC)
A miscalculation?
In the first paragraph (line 9)of this article (Greenhouse Gas) the temperature 33 degrees C does not correspond to 59 degrees F. It should be corrected. The two are related by the equation: F-32/180 = C/100. A temperature value of 33 C corresponds to 91.4 F. Neishapour (talk) 19:54, 30 October 2010 (UTC)
- The numbers refer to a difference in temperature and are therefore correct. Mikenorton (talk) 20:02, 30 October 2010 (UTC)
In other words, the increase of 33 degrees C corresponds to 91.4-32=59.4 degrees F. Because 0 degrees C equals 32 degrees F. I understand it now. Thank you. —Preceding unsigned comment added by Neishapour (talk • contribs) 20:32, 30 October 2010 (UTC)
No cause-and-effect relationship in ice-core data.
(Updated to reflect what I've been able to discover on this..)
The AGW and 'greenhouse gas' arguments hinge on ice-core data relationships between CO2 levels and temperature, yet as far as I can determine no cause-and-effect relationship between these has been established. Some published versions of the Vostok ice-core data would seem to show a small 'lead' in CO2 over temperature, suggesting a cause-and-effect relationship. However, this 'lead' seems to have crept-in in the process of conversion to svg format, the original data showing the graphs to be pretty-much synchronous.
In trying to discover whether any precise relative timing can be inferred from the original graph, the answer would seem to be... No. For example, "However, because of the difficulty in precisely dating the air and water (ice) samples, it is still unknown whether GTG concentration increases precede and cause temperature increases, or vice versa--or whether they increase synchronously." from http://www.daviesand.com/Choices/Precautionary_Planning/New_Data/
This would seem to be a key question, as without relative timing information there is no way to determine whether temperature rises are an effect, or the cause of the CO2 increase. --Anteaus (talk) 16:22, 2 December 2010 (UTC)
Natural and anthropogenic
As someone trying to understand a little about greenhouse gases - there is a table showing Natural and anthropogenic gas ppm in the atmosphere and rediative forcing. However, the table doesn't contain water vapour which seems to be the major component of greenhouse gas. It would be useful to include this so the relevance and importance of the other gases can be understood. —Preceding unsigned comment added by 210.193.162.198 (talk) 04:52, 29 June 2009 (UTC)
Shouldn't a section titled "natural and anthropogenic sources" has some tables comparing these two sources? See http://www.geocraft.com/WVFossils/greenhouse_data.html for examples, though there may be more recent or better tables elsewhere. JesseChisholm (talk) 00:04, 20 July 2010 (UTC)
- Solar emissions seems like a fundamental for "global warming" enhanced by greenhouse gasses. Where is the evidence to show the relationship? Even NASA has very little on the subject. ?--71.245.164.83 (talk) 02:43, 3 January 2011 (UTC)
First Diagram
The first diagram shows solar radiation at 343 watts per sqm. Should not this be 1366 (or something similar)? See Wikip Solar Radiation Page - http://en.wikipedia.org/wiki/Solar_radiation#Solar_constant.
- Solar radiation should be a variable. Show the history that correlates. --71.245.164.83 (talk) 02:53, 3 January 2011 (UTC)
Why the discrepancy? If there is an explanation this should be stated. Oversite22 (talk) 01:38, 16 September 2010 (UTC)
- The continuous TOA (Top of Atmosphere) value is 4 times larger than the average seen at the surface because the planet rotates. Q Science (talk) 02:22, 16 September 2010 (UTC)
The area of intercepted solar radiation is pi*r², the area of the planet's surface is 4pi*r², so the ratio is 1/4.
- ??? Yet the sun only shines on half of the earth, and the earth is round, not flat. So it is only the area exposed to earth that gets sunlight, or pi*r_squared. Its not the surface area (4"pi*r2) but the area exposed that matters. Edges of the earth receive less sunlight intensitty/area than those directly explosed. E.g. its less bright at sunrise and sunset. Where you got 4x factor is perplexing. Perhaps global cooling is a concern after all?... --71.245.164.83 (talk) 02:53, 3 January 2011 (UTC)
On the subject of diagrams, can we not find a better diagram than http://en.wikipedia.org/wiki/File:Atmospheric_Transmission.png? See the comments: http://en.wikipedia.org/wiki/File_talk:Atmospheric_Transmission.png Something like http://acd.ucar.edu/textbook/ch15/fig3.jpg would be much more relevant for explaining how GHGs work. 88.152.128.36 (talk) 09:20, 28 September 2010 (UTC)
- Sorry, can't do that because http://acd.ucar.edu/textbook/ch15/fig3.jpg is copyrighted. In addition, it only covers infrared energy leaving the planet, as seen from above the atmosphere. That said, I agree that File:Atmospheric Transmission is completely misleading and should not be used in any article. Perhaps you can create a better, more accurate, image that we can use. Q Science (talk) 19:43, 28 September 2010 (UTC)
Simple Greenhouse Diagram
The first diagram in this article shows 343 watts per square meter from source and 343 watts per square meter reflected back into space. —Preceding unsigned comment added by Multiperspective (talk • contribs) 17:27, 2 December 2010 (UTC)
- Not sure what your point is here, but for a system in thermal equilibrium, that would always be the case. Though if temperatures are slowly rising then it must be the case that radiation is less than absorbtion, but by how much I wouldn't like to say. I think it might also be added that no-one disputes the existence of the greenhouse effect, the question is whether or not small changes in CO2 concentration drive temperature changes... or whether temperature changes drive changes in CO2 levels. We simply don't know. And, since we don't know, it is bad science to claim that we do. --Anteaus (talk) 19:11, 3 December 2010 (UTC)
- I think you'll find that in the peer-reviewed literature, and in school and university level science text books, there is a complete consensus on these basic points. Fox News and a few US politicians may not have a solid grasp of the science, but that's not the science's fault, and doesn't really warrant coverage in an encyclopedia article such as this, I don't think. --Nigelj (talk) 19:28, 3 December 2010 (UTC)
Thank you for your responses. To the first, it takes a known amount of energy to increase the temperature of the average mass of the atmosphere of about 5 quadrillion tonnes (Wikipedia). This energy must be represented in any diagram depicting the mechanisms at play, either as a change in source or a decrease of albedo and/or infrared radiation measured beyond the atmosphere. There is no geologic record of this planet ever being in thermal equilibrium. To the second response. This is highly defensive and does not speak in any way to scientific discourse. In fact, repeating the mantra of "Concensus" is the antithesis of the meaning of science which at all times must include open availability of methods and raw data. —Preceding unsigned comment added by Multiperspective (talk • contribs) 00:13, 4 December 2010 (UTC)
- It does require a known amount of heat to raise the temperature a certain amount, but that is not in the drawing. The drawing shows the average values emitted by blackbodies at certain temperatures and is partly calibrated by various measurements. "The amount of heat to change the temperature" is the integral of the difference of input and output and not directly related to the values or their difference. And yes, there is consensus on that. As for "equilibrium", radiative equilibrium is obtained as soon as the temperature stops changing, typically in a few minutes, until the Sun moves to a new location or a cloud passes over. As far as the diagram is concerned, it must represent some concept of equilibrium or each day would be hotter (or colder) than the previous. There is also consensus on that. Q Science (talk) 06:58, 4 December 2010 (UTC)
"As far as the diagram is concerned, it must represent some concept of equilibrium or each day would be hotter (or colder) than the previous." This is what I am referring to. For there to be climate warming or cooling, a trend of days, years, decades, centuries,,, must be must be warmer or cooler than what preceded them. I know that sounds too simplistic but after all the modeling and calculations that is what we are talking about in greenhouse global warming or any other kind of warming or cooling, a system that is not in equilibrium. How can we have Ice Ages with sheets down to the sub-tropics and the climactic opposites in a system in equilibrium? All I am saying is that if the diagram in question is snapshot of an ideal moment of balance then that should be indicated. Being at the top of an article titled Greenhouse Gas seems inappropriate, at least without showing another depicting the net loss to space due to raising the temperature of the atmosphere. —Preceding unsigned comment added by Multiperspective (talk • contribs) 03:13, 5 December 2010 (UTC)
- I see what you're saying. If you're interested in this, I think you should research how much heat energy (in joules or watts, per square meter or for the whole planet) it has taken for "global surface temperature [to have] increased 0.74 ± 0.18 °C (1.33 ± 0.32 °F) during the 20th century." (from the Global warming article) and compare that with how much heat (in joules or watts, per square meter or for the whole planet) has arrived on the Earth from the Sun during the same period. You'll be dealing with some very big numbers, but someone must have calculated it somewhere. My guess is that the first will be a very small proportion indeed of the second figure - way too small to show up when we say "343 watts per square meter" to three significant figures. If we could find a good reference for this (without WP:OR), it would be a valuable addition to this, and maybe other articles. --Nigelj (talk) 11:27, 5 December 2010 (UTC)
- I remember it as 342 w/m^2, but last digit's uncertain. I think Kiehl & Trenberth was the one who published this figure, see page 3 of pdf.[5] About a "Simple[r]" or clearer greenhouse diagram, I think there's a better diagram that's both well referenced and clearer than the one currently on this page. --CaC 174.52.224.148 (talk) 18:58, 5 December 2010 (UTC)
Phases of Water
Water is somewhat of a unique substance that it can exist in our atmosphere in all 3 phases, solid, liquid, and gas.
I.E. Clouds & Fog are different than air in which the vapor has not condensed. Due to the droplet nature of the solid/liquid nature, refraction may also be an important aspect. Thus, water is an important aspect, both in cloudy sky and dry sky. I believe the IR spectrum is shifted with the different phases.
Should a chart be made to reflect the contribution of water on a cloudy day vs sunny day? Clouds with crystalline water, or a mix? Keelec (talk) 01:17, 18 January 2011 (UTC)
- The IR spectra of clouds approximates a black body, water vapor does not. As a result, the effect of greenhouse gases is negligible when clouds are present. However, since only about half the planet is covered with clouds, their effect is normally ignored in the radiation balance diagrams. If you can find a chart that shows the radiation balance when clouds are present, and if that chart is not copyrighted, then we should definitely include it. Q Science (talk) 20:29, 19 January 2011 (UTC)
- "The IR spectra of clouds approximates a black body". I can never understand why this is regarded as important, clouds scatter all sorts of EM radiation whether they absorb it or not. Clouds are not remotely 'a black body' since the droplets are made of water which has a refractive index >1 so any calculation based on a 'black body' assumption has got to be seriously wrong.--Damorbel (talk) 21:56, 19 January 2011 (UTC)
The Lie Of Water Vapor Being About Half Of The Greenhouse Effect.
I was surprised by some propaganda outfit calling itself "The Australian Academy Of Science" making the above claim. So naturally I wondered what gave them the "plausible deniability" to put over such an outrage on the Australian public. This is the worst lie since the USGS volcanic emissions claim.
Now I traced it back to here so far. So whats going on. If something isn't done about this I will try and get hold of Mr Wales personally. Its very clear that this is not the work of one person as when the idiot known as STOAT rigged all the usual pages. This claim is so outrageous we are going to have to trace it to a co-ordinated and probably a well-funded campaign. —Preceding unsigned comment added by 110.33.41.206 (talk) 21:36, 5 March 2011 (UTC)
- You are absolutely correct, water vapor is no where near half of the greenhouse effect. A 15C surface emits about 390 W/m2. Of that, the atmosphere absorbs about 303 W/m2, 282 W/m2 for water vapor and 21 W/m2 of CO2 (ignoring clouds and other greenhouse gases). From these numbers, it is obvious that water vapor is responsible for about 93% of the greenhouse effect. Q Science (talk) 07:46, 10 March 2011 (UTC)
Terminology
There is a current disagreement on terminology.
- ppm vs ppmv vs μmol/mol
- concentration vs mixing ratio vs mole fraction
In SI units, μmol/mol and mole fraction are the "correct" terminology. However, ppm is used in the IPCC documents and almost all the references.
The IPCC uses many phrases like
- models project atmospheric CO2 concentrations of 540 to 970 ppm - (From WG1, page 22)
At first glance, the phrase "concentration of xx ppm" is technically wrong since "concentration" represents moles per liter. However, since the number of moles in a volume is known, "concentration" can also be interpreted as "fractions of a mole per mole" (depending on the units) which is what 540 ppm means. The titles of many of the references also use the term "concentration" with this meaning. In some references, the proper phrase is "a mixing ratio of xx ppm", though "mole fraction" is also correct. Many times, AR3 uses the term "abundance", as in phrases like "with abundances reaching 2,970 to 3,730 ppb".
From the IPCC TAR Glossary
- Mixing ratio
- See: Mole fraction.
- Mole fraction
- Mole fraction, or mixing ratio, is the ratio of the number of moles of a constituent in a given volume to the total number of moles of all constituents in that volume. It is usually reported for dry air. Typical values for long-lived greenhouse gases are in the order of mmol/mol (parts per million: ppm), nmol/mol (parts per billion: ppb), and fmol/mol (parts per trillion: ppt). Mole fraction differs from volume mixing ratio, often expressed in ppmv etc., by the corrections for non-ideality of gases. This correction is significant relative to measurement precision for many greenhouse gases. (Source: Schwartz and Warneck, 1995).
In AR3, the phrase "mole fraction" is not used very often. The following is from the caption of figure 11, page 44.
- Change in CH4 abundance (mole fraction, in ppb = 10−9)
The uses of multiple phrases for the same concept indicates to me that the information is pulled from many sources and/or written by different people who use different nomenclature. At any rate, it is my opinion that changing this document to use non-standard nomenclature, even if it is "more correct", is a disservice to the readers. Q Science (talk) 20:36, 9 March 2011 (UTC)
- Thanks for providing the background here. However, since this discussion has already started in the section "Ambiguous units ppm, ppb, and ppt" at Wikipedia talk:WikiProject Chemicals, it may be better to move your text onto that page? RolfSander (talk) 20:57, 9 March 2011 (UTC)
Water vapor as greenhouse gas
It seems relevant to include water vapor in the table discussing residence time and GWP. Thanks for listening. Lfstevens (talk) 23:57, 31 December 2010 (UTC)
- Sounds like a good idea, but it may be water vapor isn't included because it is so sort lived. As stated in the Atmospheric lifetime section, water vapor "has a residence time of about nine days."--CurtisSwain (talk) 00:53, 1 January
- Show reference that water vapor has a "residence time" (citation needed) of "nine days" (show some more!) --71.245.164.83 (talk) 02:45, 3 January 2011 (UTC) (UTC)
- Placed ref in article.--CurtisSwain (talk) 06:37, 18 January 2011 (UTC)
- Show reference that water vapor has a "residence time" (citation needed) of "nine days" (show some more!) --71.245.164.83 (talk) 02:45, 3 January 2011 (UTC) (UTC)
The IPCC doesn't include WV in its list of GHGs (see any AR) because it is 'not influenced by human activity'(!). However IPCC considers WV to be a 'feedbacsk' mechanism.--Damorbel (talk) 13:01, 18 January 2011 (UTC)
Radiative forcing (W/m2)of water was added on Jan 17, 2011 and deleted soon after. Senior authors, if offended may interceed. —Preceding unsigned comment added by 118.172.36.9 (talk) 14:18, 19 January 2011 (UTC)
Lets not beat around the bush here. It has to have been a co-ordinated lie to be putting water vapor as only 36-72% of the greenhouse effect. How long has this lie been there and which group of people put this most transparent of lies into effect. No way its only one person putting this over on the public. An investigation is in order.
"Senior authors, if offended may interceed" This is of no importance, surely the inclusion or exclusion affects the science? Does anybody know how the IPCC does it calculations? --Damorbel (talk) 18:30, 19 January 2011 (UTC)
It might be short-lived, but there's always an awful lot of it in the atmosphere compared to CO2. How do we know it isn't the main cause of 'global warming'? Grassynoel (talk) 08:28, 24 February 2011 (UTC)
Grassynoel-- because the systems dictating partial pressure for water vapor in the atmosphere, due to the extremely large prevalence of liquid water on the planet, are determined in an equilibrium that changes cyclically with weather patterns but is very unlikely to change significantly overall over the timetables on which global warming has been observed. If you can find a peer-reviewed study that show that natural variations in water vapor concentration (preferably explaining through Penman equations the relationship with other climatological phenomena) explain the warming trend better than a 36% increase in CO2 concentration, this might be worth adding as a factor.66.134.4.226 (talk) 20:22, 11 April 2011 (UTC)
Water vapour, the manipulated and the controlled variables (MV and CV)
There seem to be three concurrent discussions here on aspects of water vapour, so rather than pick one, I thought I'd start another :-) When you deal with a complex feedback system, it is important to sort out the inputs from the outputs. In this case, gas concentrations that humans can do something about are the inputs. The extent of the greenhouse effect, measured as a temperature anomaly, a change in energy-transfer rate, or whatever, is the output of the system. The concentration of water vapour is part of one of the feedback mechanisms (the warmer the atmosphere, the more water vapour it can (and will) hold, etc). Therefore, we must not confuse it with the inputs or the whole model becomes impossible to understand. If there were millions of people spraying water mist into the air somewhere, we might consider it as an input, but they are not and so that is not helpful.
It would not matter if it were a proven fact that 90% of the greenhouse effect were caused by water vapour, what follows would still be true. The process is that people alter the concentration of a greenhouse gas like CO2; this causes the earth and the atmosphere to begin to warm; the warmer air causes more water from the oceans to evaporate; the extra water vapour in the air accelerates the initial warming started by the input gas; eventually a new equilibrium would be reached where the total effect of that perturbation in CO2 concentration could be measured as an output. In practice, we never could get to the bottom of it that way as there are other feedbacks going on, such as changes in ice albedo, as well as not one but continual emissions of not just CO2 but a whole slew of greenhouse gasses.
That, I understand, is why the IPCC and others do not either count H2O as a greenhouse gas, nor do they run around in a panic when the popular press point out that they seem to have forgotten it. We're here to help people understand the very hard stuff that scientists are working on, not to act as an echo chamber for those who do not. --Nigelj (talk) 20:32, 6 March 2011 (UTC)
Those of us that are capable of intelligent, independent thought and critical thinking can plainly see the real reason why the IPCC and others do not count the most prevalent greenhouse gas in their calculations. The corruption on this issue is clear-cut, obvious, and disgusting. The scientific community should be ashamed of themselves for misleading the public on this issue, and you WILL be exposed. Those arguing on the side of the IPCC are either part of the corruption or easily influenced and incapable of critical thinking. I have read every IPCC report and the assumptions that models are based on are absurd. Correct scientific method suggests that to claim a "consensus" on a subject, by those in the scientific community, is in itself, proof of corruption. You people need to take a good, hard look at yourselves, and ask - "Am I a scientist, or a politician?” Charles Darwin would be rolling in his grave. Pahgcdt (talk) 00:46, 5 April 2011 (UTC)
Pahgcdt-- You clearly have strong opinions, and yet an extraordinary deficit of evidence. Every claim of scientific malfeasance has been thoroughly refuted, every claim of error admitted or addressed. Your personal belief that the scientific consensus on global warming is some kind of elaborate hoax is amusing and kind of cute, but has no place on this discussion page, as this is not a forum. If you really think that the Democratic party invented the greenhouse gas effect for purposes of self-enrichment, despite the fact that the greenhouse gas effect was discovered four years before the Democratic party was even founded, then by all means go out and prove it, get it peer-reviewed, and maybe there can be a "Nefarious Conspiracy" section that references your very own paper. Until then, unless you have a specific improvement to the article's discussion of water vapor as a feedback mechanism driving the greenhouse effect, I must instead refer you to www.redstate.com.66.134.4.226 (talk) 20:33, 11 April 2011 (UTC)
Radiation Transmitted by the atmosphere
The third chart here is very interesting. Does anybody know its source? It raises a question. It appears that the effect of CO2 has reached sort of a spectral saturation in the infrared band. That is, another 10% increase in CO2 will not increase the absorbtion of infrared, because radiation in the CO2 band is already 100% absorbed. Can somebody tell me what I'm missing here? Stevevogelnu (talk) 01:00, 13 March 2011 (UTC)
- That is one of the skeptic arguments. One counter argument is that the edges of the absorption bands have a slight slope. As a result, increasing CO2 will make the bands slightly wider. There are additional arguments to counter that, and so forth. Q Science (talk) 17:19, 13 March 2011 (UTC)
The impression that I draw from your answer is that global warming is an extremely subtle affect that is not well understood. Stevevogelnu (talk) 13:51, 14 March 2011 (UTC)
- Actually, this effect is quite well understood, and saturation of the CO2 absorption bands is not occurring in the thin, upper atmosphere, which is where the absorption is most important. See the following descriptions at RealClimate and Skeptical Science. Parejkoj (talk) 14:37, 14 March 2011 (UTC)
- The realclimate article (which you linked to twice) claims that "higher layers ... are colder layers, so they do not radiate heat as well." Apparently, these scientists have never heard of the stratosphere. As a result, they have missed a major negative feedback. The realclimate article also ignores the fact that most of the energy absorbed by CO2 is absorbed within 20 meters of the surface. Sorry, but that is not the cold upper atmosphere. Q Science (talk) 21:19, 14 March 2011 (UTC)
- I fixed the Skeptical Science link. I should have also linked their more advanced version. Please read some more on how the greenhouse effect works. What's important is not the absorption near the surface, but rather absorption in the mid-to-upper troposphere that affects the radiation balance at the top of the atmosphere. Some other references for this are Wikipedia's own page, and the included references and this page, and included references. Parejkoj (talk) 15:21, 15 March 2011 (UTC)
- The skepticalscience articles are based almost entirely on a mis-interpretation of the upwelling spectra. The realclimate explanation relies heavily on Annan and Hargreaves. These researchers explain the entire temperature rise since the last glacial maximum as being caused by CO2 and ice feedback. Apparently, they have never heard of the Milankovitch cycles. I am beginning to think that the skeptics might have a point. Q Science (talk) 05:30, 16 March 2011 (UTC)
GHG intensities and other changes
I've revised the section on regional and national attribution of emissions. My main concern was the WRI GHG intensity figure which was previously included without explanation. I've added an explanation of some of the issues involved in calculating net emissions and GHG intensities.
I've rewritten the bit on cumulative emissions. In the previous revision, I made the mistake of assuming the IEA's assessment was a ranking of all countries'/regions' emissions. The IEA report does not actually state this, therefore, to present the data as a ranking is possibly misleading, i.e., some countries/regions not included by IEA may rank higher than the countries they did include. Enescot (talk) 14:50, 11 April 2011 (UTC)
Water Vapor and runaway warming, statement based on Held (2000)
This comment is in relation to this edit and revert
The citation (Held, 2000) is not specific, so I'm guessing the citation is supposed to point to a paragraph near the top of page 449 that discusses a runaway situation. I don't believe the author was discussing a runaway change within the tolerance of a liquid water earth. Its my belief the paper is referring to a runaway venus syndrome (see his cite #22 for example). Further, while the paper did say its self evident a runaway situation isn't happening now, I think the author was suggesting it could at higher temps. Again, I think the author was talking in context of venus syndrome, not liquid-water earth climate. Our sentence in the article is talking about today's liquid-water earth climate, so if I'm reading the paper correctly, then this entire sentence needs alternative citation or deletion. NewsAndEventsGuy (talk) 10:57, 9 June 2011 (UTC)
- Alternatively, I suppose, the sentence in the article could be modified to clarify the scope of "runaway" being talked about. Until recently, I never knew there was a theory for runaway by which we could vaporize the oceans. I thought it just meant that positive feedbacks would runaway to the top of the curve, before negative feedbacks (or large external forcing) kicked in to go the other way. I'm guessing most readers will understand the term the same way. So if the sentence in the article means venus syndrome, this could also be resolved consistent with the quoted source by clarifying the sentence context in the article.NewsAndEventsGuy (talk) 11:19, 9 June 2011 (UTC)
- Regardless of what our runaway global warming article says, in the scientific literature the phrase almost exclusively has referred to what you call above the runaway venus syndrome; that is, a positive water vapor feedback that results in the complete evaporation of a planet's liquid water oceans. Although there's nothing special about water. It could also be any other greenhouse gas that is in equilibrium with a liquid ocean of the same substance. -Atmoz (talk) 17:35, 12 June 2011 (UTC)
- Yes I see that now. However, wiki is not an academic or scientific journal so as editors we should write for the lay audience who may be doing cursory reading. That means not assuming the reader is using either the scientific definition or the MSM definition given to the term. Instead, we should add text so the meaning is clear at first reading.NewsAndEventsGuy (talk) 11:37, 13 June 2011 (UTC)
- While we should define exactly what is meant by runaway global warming, we should most certainly not "redefine" it to cater to a lay-audience. --Kim D. Petersen (talk) 12:41, 13 June 2011 (UTC)
- I agree, but I think you missed my point. My point is that where we want to write about "process X" and we want to use technically precise language, we run a risk. The risk is that the technically precise language might also be used WRONG in the main stream media and the lay reader might have no idea there's a difference. (Take me for instance, I've been reading 25 years of MSM on climate change and only just learned about the technically precise meaning of "runaway climate change".) We can either assume the lay reader will instantly magically figure out that MSM uses technically precise language wrong, or we can help educate the lay reader by paying attention when we use terms the MSM uses wrong. IMO, at such moments in our writing using the technically precise term and just blundering ahead will perpetuate lay reader confusion and ignorance, whereas taking a moment to to help the reader avoid this trap will make our writing more effective. NewsAndEventsGuy (talk) 16:05, 13 June 2011 (UTC)
- Even if it is used wrongly in the media, then we still use the technically precise language here. Wikipedia does not use neologisms and other incorrect language, just because it is used in other places. Nothing hinders us in presenting a lay-readable presentation of a topic and still use correct terminology. If a wording can create a wrong impression, then either describe the term in-line, give a footnote, or wikilink to a correct description. --Kim D. Petersen (talk) 17:44, 13 June 2011 (UTC)
- I agree, Kim! Indeed, if editors had followed your advice to make an inline explanation it would have averted this very thread!
- Even if it is used wrongly in the media, then we still use the technically precise language here. Wikipedia does not use neologisms and other incorrect language, just because it is used in other places. Nothing hinders us in presenting a lay-readable presentation of a topic and still use correct terminology. If a wording can create a wrong impression, then either describe the term in-line, give a footnote, or wikilink to a correct description. --Kim D. Petersen (talk) 17:44, 13 June 2011 (UTC)
- I agree, but I think you missed my point. My point is that where we want to write about "process X" and we want to use technically precise language, we run a risk. The risk is that the technically precise language might also be used WRONG in the main stream media and the lay reader might have no idea there's a difference. (Take me for instance, I've been reading 25 years of MSM on climate change and only just learned about the technically precise meaning of "runaway climate change".) We can either assume the lay reader will instantly magically figure out that MSM uses technically precise language wrong, or we can help educate the lay reader by paying attention when we use terms the MSM uses wrong. IMO, at such moments in our writing using the technically precise term and just blundering ahead will perpetuate lay reader confusion and ignorance, whereas taking a moment to to help the reader avoid this trap will make our writing more effective. NewsAndEventsGuy (talk) 16:05, 13 June 2011 (UTC)
- While we should define exactly what is meant by runaway global warming, we should most certainly not "redefine" it to cater to a lay-audience. --Kim D. Petersen (talk) 12:41, 13 June 2011 (UTC)
- Yes I see that now. However, wiki is not an academic or scientific journal so as editors we should write for the lay audience who may be doing cursory reading. That means not assuming the reader is using either the scientific definition or the MSM definition given to the term. Instead, we should add text so the meaning is clear at first reading.NewsAndEventsGuy (talk) 11:37, 13 June 2011 (UTC)
- Bear in mind that the precise moment in time a neologism crosses into the accepted vernacular and therefore requires disambiguation is a blurry line, over which reasonable minds can differ. Its my guess if you google-scholar search [geoengineering runaway "global warming" -venus] you'll agree there are so many instances in various non-climatological journals that this term needs disambiguation (or the equivalent). NewsAndEventsGuy (talk) 19:05, 13 June 2011 (UTC)
Delete lede 2nd paragraph?
If this article is attempting to talk about greenhouse gasses on any planetary body as a general principle of physics, as distinct from a focus on earth's paleo or contemporary greenhouse gasses, then it strikes me as odd to have a lead paragraph that is not only earth-specific, but an earth-CO2 specific paragraph in the lead. Seems to me that this info could go in subsections in this article, or even better maybe it would be appropriate to split the article between "greenhouse gasses" (talking about general principles applicable to the gasses on any planetary body) and "greenhouse gasses (Earth)" talking about them on our specific orb. NewsAndEventsGuy (talk) 18:04, 27 June 2011 (UTC)
Potency claim for CH4 versus CO2
Article claims that as a greenhouse gas, CH4 is eighty times more potent on a molecule to molecule basis, according to reference 8. I followed citation given for reference 8, and the link I arrived at is below.
http://iopscience.iop.org/0034-4885/68/6/R02/
I downloaded the .pdf, and all I can find is a statement on pg 1362 that states CH4 is EIGHT times more potent.
If the author can show where the "eighty" statement came from it should stay, otherwise the potency should be decimated.
Stevew139 (talk) 08:54, 9 June 2011 (UTC)
- I replaced that citation with the IPCC cite used later in the article for the same factoid, and modified the text according to what IPCC AR4 said, which was, that over 20 years methane has 72x the warming potential of CO2. If has a much lower potential over a 100 year time frame. (If we're only talking about the methane itself, and not the warming due to indirect effects of the methane in the atmosphere.) NewsAndEventsGuy (talk) 02:10, 29 June 2011 (UTC)
Outline organization
Today I attempted to sort out what I viewed as a jumbled up organization of the text with respect to section headings. IMO that task is not yet done, but I'll stop for now to let other editors chime in. If you want to do more, great, and if you think my reorganization attempt was misguided, revert is welcome but please explain why here in TALK. NewsAndEventsGuy (talk) 17:18, 11 July 2011 (UTC)
Potency claim for CH4 versus CO2
Article claims that as a greenhouse gas, CH4 is eighty times more potent on a molecule to molecule basis, according to reference 8. I followed citation given for reference 8, and the link I arrived at is below.
http://iopscience.iop.org/0034-4885/68/6/R02/
I downloaded the .pdf, and all I can find is a statement on pg 1362 that states CH4 is EIGHT times more potent.
If the author can show where the "eighty" statement came from it should stay, otherwise the potency should be decimated.
Stevew139 (talk) 08:54, 9 June 2011 (UTC)
- I replaced that citation with the IPCC cite used later in the article for the same factoid, and modified the text according to what IPCC AR4 said, which was, that over 20 years methane has 72x the warming potential of CO2. If has a much lower potential over a 100 year time frame. (If we're only talking about the methane itself, and not the warming due to indirect effects of the methane in the atmosphere.) NewsAndEventsGuy (talk) 02:10, 29 June 2011 (UTC)
Outline organization
Today I attempted to sort out what I viewed as a jumbled up organization of the text with respect to section headings. IMO that task is not yet done, but I'll stop for now to let other editors chime in. If you want to do more, great, and if you think my reorganization attempt was misguided, revert is welcome but please explain why here in TALK. NewsAndEventsGuy (talk) 17:18, 11 July 2011 (UTC)
Atmospheric Lifetime
The following statement in section 6.2 contradicts the preceding definition of lifetime and should be deleted: "The atmospheric lifetime of CO2 is often incorrectly stated to be only a few years because that is the average time for any CO2 molecule to stay in the atmosphere before being removed by mixing into the ocean, photosynthesis, or other processes. However, this ignores the balancing fluxes of CO2 into the atmosphere from the other reservoirs. It is the net concentration changes of the various greenhouse gases by all sources and sinks that determines atmospheric lifetime, not just the removal processes." If the inputs are included to balance out the outputs, then the lifetime for any gas with a stable concentration would equate to infinity (divide by zero). This is clearly wrong and it is the flux or flow of the substance through the atmosphere which is the denominator not the difference between the inputs and outputs. For a stable concentration the input equals the output, but as per water vapor, that does not mean that it has an infinite lifetime. This misunderstanding has propagated numerous incorrect statements such as "Recent work indicates that recovery from a large input of atmospheric CO2 from burning fossil fuels will result in an effective lifetime of tens of thousands of years." in the following section. It is quite apparent from the amplitude of the yearly CO2 concentration cycle that it's Lifetime is relatively short. The only way it could get to tens of thousands of years would be in situations like "snow ball earth" with no plants and no plankton etc. I suggest the above statement be replaced with "The atmospheric lifetime of CO2 is often incorrectly stated to be thousands of years or more, due to substituting the net input minus output flux through the atmosphere in place of the output flux. The atmospheric lifetime of CO2 is actually only a few years because that is the average time for any CO2 molecule to stay in the atmosphere before being removed by mixing into the ocean, photosynthesis, or other processes." — Preceding unsigned comment added by BFhybrid (talk • contribs) 13:13, 7 June 2011 (UTC)
- The correct definition of atmospheric lifetime in the context of greenhouse gases is defined by what the literature states, and not what we as editors judge to be the correct definition. I've spent a bit of time looking in the TAR for its definition (which can be found here), and that one contradicts your personal definition of molecule lifetime, and is similar to the definition in 6.2. So that is the one that the preponderance of the literature seems to support. If you disagree, then i suggest that you find sources of equivalent merit to support yours. (suggestion: The U.S. Global Change Research Program or the AR4 might be a place to look) --Kim D. Petersen (talk) 15:59, 7 June 2011 (UTC)
- The definition that I am discussing is not 'my personal definition' as you make out, it is the clear definition that is already referenced in 6.2 to Jacob, and includes the equation with explanations of each variable; maths is the universal language and it is unambiguous. I found no definition of atmospheric lifetime in your link, only one oblique reference to lifetime, in the obvious statement that well mixed gases have longer lifetimes. You have not addressed my objection that the second paragraph is contradicting the first paragraph, and the statement you are supporting is already tagged with 'citation needed'. What I don't understand is your claim that the definition you found in the TAR (what is this?) is similar to the definition in 6.2. Perhaps you could just extract the definition you are referring to from the TAR and post it here? Here is the first internet reference I found on Google on this issue [1] BFhybrid (talk) 12:47, 8 June 2011 (UTC)
- BFhybrid, you are absolutely correct. But I caution you not to pursue this matter because you will just get more frustrated. If you go back into the archives you'll see contributions from me on this subject. Physicists and engineers use half-lifes to describe exponentially decaying processes, and don't generally use the simple term "lifetime" except in casual conversation. Authors writing about greenhouse gas/effect make up their own definitions; you won't find a consistent definition. Indeed if someone attempted to create one, it would be torn to shreds or just ignored by others who have preconceived notions of what the end result should be. I've read some of the papers that assert lifetimes of the order of 100,000 years for CO2 in the atmosphere. While they may make some attempt to descibe how they arrive at a figure, they're not precise in describing where they set the concentration threshold on the way to an infinitely long lifetime. As for my understanding of the matter, when the terms are shifted to those physicists would use, the half-life of CO2 in the atmosphere (sources cease, decay only) is somewhere near three months (if I remember correctly). The more interesting observations that can be made are about the mechanisms. CO2 removal is a largely a surface effect, depending on how you view H2O condensation and the mass transport of CO2 to the surface by precipitation. H2O removal is a volume effect for the most part. Water vapor condenses at temperatures commonly found in the atmosphere; CO2 does not change state at temperatures found in the atmosphere. These are the underlying reasons why CO2 leaves the atmosphere far more slowly than H2O. blackcloak (talk) 09:37, 5 August 2011 (UTC)
Resource data ...
Resource for comparative data on carbon emissions in the United States, China, and India from the United States Energy Information Administration ... http://www.eia.gov/oiaf/ieo/pdf/ieorefcase.pdf 216.250.156.66 (talk) 20:18, 28 July 2011 (UTC)
History of scientific research
There is a link in this section ( http://www.oppapers.com/essays/Global-Warming-Opposing-Viewpoints-Paper/733173?topic ) with a pay wall, it is to a purely commercial organisation trying to sell "teach you how to write". There is no access to samples of what they do or anything of that sort and it certainly does not have anything to say about GH gases unless you pay first. I will be removing this shortly unless a good argument is presented. --Damorbel (talk) 11:08, 12 September 2011 (UTC)
- I created this link in this edit today. Previously the reference was "Opposing Viewpoints Resource Center. Detroit: Thomson Gale, 2005. From Opposing Viewpoints Resource Center" with no links. I have no idea who or what 'Opposing Viewpoints' is either. The problem is that the statements it provides a source for are self-evidently true. What we need is a better reference for this level of historical summary. --Nigelj (talk) 16:49, 12 September 2011 (UTC)
Typo in image
The image on the page has a typo in the bottom left-hand corner: "Absorbation" - instead of "Absorption"?
File:The_green_house_effect.svg
Can we fix it?
Drdrperry (talk) 00:22, 15 September 2011 (UTC)
Associated Press resource
Biggest jump ever seen in global warming gases by Seth Borenstein, Associated Press (via USA Today) ... excerpt "The global output of heat-trapping carbon dioxide jumped by the biggest amount on record, the U.S. Department of Energy calculated, a sign of how feeble the world's efforts are at slowing man-made global warming." 99.109.125.146 (talk) 22:42, 3 November 2011 (UTC)
Question about conversions
Sorry if posting this in the wrong spot.
Isn't 59 deg F = 15 deg C?
Seems all the C to F conversions are off.
Brehart (talk) 10:02, 15 November 2011 (UTC)
- Are you referring to the footnote that reads "Note that the greenhouse effect produces a temperature increase of about 33 °C (59 °F)? If so, it's correct. An increase in 33C is the same as an increase of 59.4F. (33/5 *9). It's about the change in temperature, not a specific temperature.VsevolodKrolikov (talk) 10:18, 15 November 2011 (UTC)
Understood (thanks for clarifying). Seems the average consumer would not read it that way, but makes sense now that I re-read it with your explanation.
Brehart (talk) 18:45, 15 November 2011 (UTC)
"Absorbs and emits"?
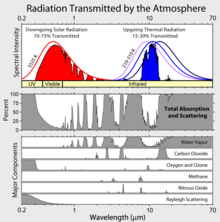
The article begins, "A greenhouse gas (sometimes abbreviated GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range." Why do we have "emits" in there? The characteristic of a GHG is the existence of absorption bands in the IR region of the spectrum equivalent to the surface temperatures of the planet, so absorb is definitely right.
Does not any gas emit IR radiation equivalent to its blackbody temperature? I may not be correct here, as other gasses will be transparent and I'm not sure if transparent things emit IR like blackbodies, so this really is a question, not yet a suggestion and certainly not a criticism. Whichever is right, I didn't find the answer in the article. Maybe I missed it, or maybe we can improve something here. --Nigelj (talk) 18:49, 2 October 2011 (UTC)
- The current phrasing is correct. Not all gases emit in the infrared when heated. For example, if you were to look at a heat gun through an infrared camera, you would not be able to see anything leaving the gun. This is because the primary components of air (oxygen and nitrogen) do not emit radiation in the infrared. However, if you were to introduce CO2 into the heat gun intake, you'd very clearly see it show up on the infrared display because CO2 does emit in that range. So it's very much correct to make the distinction that only some gases absorb energy and emit in the infrared. --Sean 130.253.30.70 (talk) 21:38, 2 October 2011 (UTC)
- You've stumbled into an area that has been discussed many times in the past. The current dumbed down wording is intended to provide the general reader with a simple statement about the critical link between thermal energy storage in the atmosphere and IR sources (absorption) and sinks (emission/re-radiation) of energy. The intro is not intended to be technically accurate, literate or complete. To get into some of the technicalities, even though these ideas do not necessarily belong in an introduction, I do not believe there are any molecular species found commonly in the atmosphere that exhibit phosphorescence or phosphorescence. It is a general rule that at any given wavelength, the absorption coefficient and the emissivity coefficient are equal, which simply means the a good absorber is an equally good emitter. What is missing in the intro is the idea that the non-GHG components (about 99%) of the atmosphere are the actual thermal reservoir (which we feel as the air temperature) and that GHG concentration controls the rate at which energy moves both into and out of the atmosphere (for a given temperature of the atmosphere and IR field strength). The problem, from a writer's point of view, is which facts need to be touched upon in the intro in order to give the reader enough to begin to understand the scope of the article. blackcloak (talk) 06:08, 26 October 2011 (UTC)
- The atmosphere absorbs from a hot surface during the day and emits different frequencies at night (because the air is colder than the surface was). As I understand it, that meets all the requirements of phosphorescence.
- Emitted frequency is lower than absorbed frequency
- Delay between absorption and emission is greater than 1ms
- Thus, the atmosphere itself is phosphorescent. Q Science (talk) 06:41, 26 October 2011 (UTC)
- No, not phosphorescent. Phosphorescence, as far as I know, does not occur in gases. If you check the Wiki article it refers to the delay in re-emission characteristic of phosphorescence being "associated with "forbidden" energy state transitions in quantum mechanics", these transitions can only occur in solid materials. The IR absorption/emission associated with the so-called greenhouse gases is due to molecular vibrations that are directly related to the molecular temperature. Phosphorescence is not particularly dependent on temperature, whereas the absorption/emission of GHGs is an exact function of the temperature of the molecule.--Damorbel (talk) 09:10, 26 October 2011 (UTC)
- The atmosphere absorbs from a hot surface during the day and emits different frequencies at night (because the air is colder than the surface was). As I understand it, that meets all the requirements of phosphorescence.
- That explains how it works in some solids. From The Free Dictionary
- Persistent emission of light following exposure to and removal of incident radiation
- From Merriam-Webster
- luminescence that is caused by the absorption of radiations (as light or electrons) and continues for a noticeable time after these radiations have stopped
- From dictionary.reference.com
- any luminous radiation emitted from a substance after the removal of the exciting agent
- a fluorescence for which the average lifetime of the excited atoms is greater than 10--8 seconds
- The point of these is that
- Radiation causes the effect
- Emission continues for a noticeable time after the forcing radiation stops
- BTW, to create a laser, the lasing material must be phosphorescent. That is true of solid, gas, and dye (liquid) lasers. HeNe and CO2 lasers are both fairly common gas lasers. Q Science (talk) 13:17, 26 October 2011 (UTC)
- "to create a laser, the lasing material must be phosphorescent" Really? It doesn't mention this in the Wiki laser article. And a solid state laser's output is proportional to the input current (above the threshold). Also a laser can be modulated very quickly (many MHz) to give femtosecond pulses, which is rather different from phosporescent material, which can glow for an hour or more after the stimulus is withdrawn. --Damorbel (talk) 17:47, 26 October 2011 (UTC)
- That explains how it works in some solids. From The Free Dictionary
- Well, we all know better than to trust wikipedia. (Grin) The trick is that if the material can store energy long enough, then it is possible to stimulate an emission and, by definition, any material capable of storing that type of energy for that long is phosphorescent. In some cases, the energy can be converted (via stimulated emission) into light in a femtosecond. Typically, the lasing wavelength is not the same as the pumping wavelength, another characteristic of phosphorescence. Q Science (talk) 18:00, 26 October 2011 (UTC)
- Phosphorescence is not coherent, but stimulated emissions are. Q Science (talk) 21:14, 26 October 2011 (UTC)
- Re. - the line above Why then does, as you write, "the lasing material must be phosphorescent"? As noted Phosphorescence is a process whereby the emission is delayed minutes, hours and days; whereas lasing is in the femtosecond region. Do you not see any difference? Phosphorescence does not involve stimulated emission so it doesn't have anything to do with "light Amplification by Stimulated Emission of Radiation, LASER for short. Phosphorescence and lasing are very different (physical) processes, producing very different results, in any reasonable encyclopedia they should not be confused. --Damorbel (talk) 09:10, 27 October 2011 (UTC)
- Phosphorescence is not coherent, but stimulated emissions are. Q Science (talk) 21:14, 26 October 2011 (UTC)
- Spontaneoous emission from a triplet state occurs very slowly, by comparison with fluorescent transitions, and is called phosphorescence.
- Molecular fluorescence is responsible for dye laser emission.
I was not aware that dye lasers were based on fluorescence. I have not been able to find many other references. The following reference supports the idea that the lasing medium is phosphorescent.
Perhaps you can find additional references. It appears that we need to add this to the laser and stimulated emission articles. Q Science (talk) 14:05, 28 October 2011 (UTC)
- Both your refs. state that laser action arises from stimulated emission with the atoms (or molecules) in a highly excited state, also described as population inversion. With no stimulation some excited material may emit by slow process such as fluorescence and phosphorescence but there are plenty of ways other than these two to get the necessary population inversion, semiconductors can lase if driven hard enough but they are not phosphorescent; gas discharges do the same thing in the He/Ne laser but you will not get He/Ne to phosphoresce or fluoresce. I think you are just naming all light emitting processes 'phosphorescence' or 'fluorescence', which is not exactly scientific. The article on luminescence may help you to sort out the differences in the various sorts of light emission. Read the link and all the links in the article - you will then discover what a complicated subject the interaction of light and matter is; the terminology needs to be used precisely if confusion is to be avoided. --Damorbel (talk) 21:12, 30 October 2011 (UTC)
I agree; how arrogant can you be? You know a couple of characteristics of phosphorescence and so anything that has those two characteristics must be phosphorescent? Stores energy and releases it later. So, you would argue that a bouncing spring exhibits "a form of phosphorescence"? A Thermos flask? You need to understand that when a physicist speaks of things like lasers as "a form of phosphorescence", he or she does so because they know that "phosphorescence" is something that most people think they understand, or are familiar with. They have to refer to it in simple, familiar-but-not-technically-correct terms so people like us can understand the concepts at work, even if that understanding is extremely limited and so grossly oversimplified that it causes not just physicists but ordinary people like me to have to tell you to please stop embarrassing yourself by arguing about things you so very clearly have very much a layman's "casual understanding" of. Maybe read Wikipedia a bit more, and write a bit less. (124.168.202.62 (talk) 20:33, 28 January 2012 (UTC))
Introduction and recent growth in emissions
I've rewritten the introduction of the article. I'll outline what I view to be its problems below. Old revision:
- Since the beginning of the Industrial Revolution, the burning of fossil fuels has contributed to the increase in carbon dioxide in the atmosphere from 280ppm to 390ppm, despite the uptake of a large portion of the emissions through various natural "sinks" involved in the carbon cycle.[5][6] Carbon dioxide emissions come from combustion of carbonaceous fuels such as coal, oil, and natural gas. CO2 is a product of combustion of carbon although burning coal for example, also produces carbon monoxide.[7] {{Since 2000 fossil fuel related carbon emissions have equaled or exceeded the IPCC's "A2 scenario", except for small dips during two global recessions.[8][9][10] In 2010, global CO2 emissions exceeded the IPCC's worst case scenario,[11] leading to concerns over whether dangerous climate change can be avoided.[12]}}
The part in braces is what I have a problem with:
- {{ (1) Since 2000 fossil fuel related carbon emissions have equaled or exceeded the IPCC's "A2 scenario", except for small dips during two global recessions.[8][9][10] (2) In 2010, global CO2 emissions exceeded the IPCC's worst case scenario,[11] leading to concerns over whether dangerous climate change can be avoided.[12]}}
The SRES A2 scenario is not something most people will be familiar with, so mentioning it without explanation is not particularly helpful. Also, I feel that recent emissions growth is not sufficiently important to be mentioned in the introduction, at least in this form. Sentence (2) is really not that important, in the sense of one year's emissions exceeding a particular IPCC scenario. The scenario is described as "worst-case", which lacks objectivity, e.g., emissions growth has also corresponded in some regions with increases in income, which I personally do not see as a bad thing. The second subject of (2) concerns "dangerous" climate change, which is not defined. Since there is no objective definition of "dangerous" climate change, referring to it without explanation is, in my view, not appropriate. My new revision (in braces):
- (as per existing revision) Since the beginning of the Industrial Revolution, the burning of fossil fuels has contributed to the increase in carbon dioxide in the atmosphere from 280ppm to 390ppm, despite the uptake of a large portion of the emissions through various natural "sinks" involved in the carbon cycle.[5][6] Carbon dioxide emissions come from combustion of carbonaceous fuels such as coal, oil, and natural gas. CO2 is a product of combustion of carbon although burning coal for example, also produces carbon monoxide.[7]
- {{The governments of most countries in the world[2] have agreed that the future increase in global mean temperature (i.e., global warming) should be limited to below 2 °C relative to the pre-industrial temperature level, and would require deep cuts in global GHG emissions from their present level.[3] Analyses by the United Nations Environment Programme (published in 2011)[4] and International Energy Agency (2011)[5] suggest that current climate change policies to reduce emissions are inadequately stringent to meet the 2 °C target at reasonable cost.}}
Enescot (talk) 17:36, 25 November 2011 (UTC)
- What's going on here? The intro now has all this stuff about global warming. This is not an article about that subject. Why is no one removing this material- or at least placing it in its proper place? blackcloak (talk) 20:01, 5 December 2011 (UTC)
- I agree. The third paragraph in particular has no place in this article. Plantsurfer (talk) 21:21, 5 December 2011 (UTC)
- I think "para 3" started here [6]. I agree: there is now too much of it. Only a brief mention is appropriate William M. Connolley (talk) 21:53, 5 December 2011 (UTC)
- Agree also. If someone changes the subject, I have to question whether further information is reliable, regardless of whether I agree with them or not. I also don't want to have to sift through an article to glean the pertinent parts. — Preceding unsigned comment added by 65.102.59.64 (talk) 23:32, 5 December 2011 (UTC)
- Deleted paragraph 3 of intro as per discussionPlantsurfer (talk) 23:42, 5 December 2011 (UTC)
- I am uncomfortable with para 2 of the introduction because it launches into a statement about post-industrial CO2 rise without providing any background information about earth's atmospheric composition in the past. That is required for context and balance. Plantsurfer (talk) 00:03, 6 December 2011 (UTC)
Addition of current graphs/charts
Hello, I noticed that many of the images used in the article only included information up to 2000. Being as it is now 2012, are there current images that can be used in the article to demonstrate the points made? Thank you. Lucyburb (talk) 20:19, 21 January 2012 (UTC)
Edit request on 14 March 2012
This edit request has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
Can I edit.
120.50.183.201 (talk) 11:16, 14 March 2012 (UTC)
- Unfortunately, the article is currently semi-protected, so that only established users may edit it (it has been the target of heavy vandalism). However you can request an edit be made, if you see something wrong with the article. Describe it, and the regular editors of this page will take a look. Hope this helps, --TeaDrinker (talk) 11:19, 14 March 2012 (UTC)
Lifetime of gases
A quick read of ornl.gov suggest decades may be correct. CO2 is 100 years, NO2 is 114 years. Hardly centuries. One gas SF6 is 3200 years. Jim1138 (talk) 17:47, 1 June 2012 (UTC)
- Perhaps you could read more slowly http://www.realclimate.org/index.php/archives/2005/03/how-long-will-global-warming-last/ William M. Connolley (talk) 21:18, 1 June 2012 (UTC)
- The U.S. Environmental Protection Agency reference given in RealClimate is out of date and is now a 404 link, but I have tracked down the current report at [7] and added a ref onto this sentence. For CO2 the report clearly says, "Atmospheric lifetime (years) 50-200" with the footnote, "No single lifetime can be defined for CO2 because of the different rates of uptake by different removal processes", just like RC said it did. --Nigelj (talk) 00:23, 2 June 2012 (UTC)
- Ooh, actually, RC says 5-200 years, but the current report clearly says 50-200 years. I don't know if this was a misprint in RC, or an update by the US EPA. --Nigelj (talk) 00:26, 2 June 2012 (UTC)
IPCC citations
I'm thinking of changing the IPCC citations in this article. I would change the citations so that they use the harvnb template as is done in effects of global warming. The change would remove a lot of repetitive information contained in the existing IPCC citations. I'd probably make the changes gradually, perhaps revising a few citations in each edit. Enescot (talk) 21:05, 17 July 2012 (UTC)
Biased summary of climate change mitigation policies
I've removed the following two paragraphs from the section on "Anthropogenic greenhouse gases":
- The US Environmental Protection Agency (EPA) ranks the major greenhouse gas contributing end-user sectors in the following order: industrial, transportation, residential, commercial and agricultural.[45] Major sources of an individual's greenhouse gas include home heating and cooling, electricity consumption, and transportation. Corresponding conservation measures are improving home building insulation, installing geothermal heat pumps and compact fluorescent lamps, and choosing energy-efficient vehicles.
- [...] On December 7, 2009, the US Environmental Protection Agency released its final findings on greenhouse gases, declaring that "greenhouse gases (GHGs) threaten the public health and welfare of the American people". The finding applied to the same "six key well-mixed greenhouse gases" named in the Kyoto Protocol: carbon dioxide, methane, nitrous oxide, hydrofluorocarbons, perfluorocarbons, and sulfur hexafluoride.[47][48]
I do not see why this article should emphasize emission sources and policies in the US. This article should take a global perspective. I've rewritten the section so that it focuses on the UNFCCC. Enescot (talk) 20:39, 18 July 2012 (UTC)
Diagram of greenhouse effect

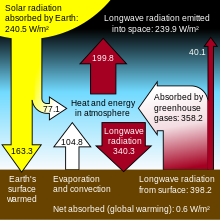
In the lede, I've swapped the diagram of the greenhouse effect for another version. The old version has two problems: the first is that the figures used in the diagram are unsourced. The second is that there is poor contrast between some of the colors used in the diagram. This makes the diagram less suitable for people who are visually impaired. Enescot (talk) 04:55, 22 October 2012 (UTC)
Too difficult to understand
I read the following sentence five times, and I can't figure out how it is saying what I know it is trying to say.
"Carbon monoxide has an indirect radiative effect by elevating concentrations of methane and tropospheric ozone through scavenging of atmospheric constituents (e.g., the hydroxyl radical, OH) that would otherwise destroy them."
Too much is trying to be said in too few words. What good is it to be terse while being obtuse? Clarifying who "them" is would help greatly, but, more to the point, introducing the general point of scavenging, providing the example of an injected CO component into the atmosphere where OH occurs naturally, and concluding with the radiative implications would guide the reader. blackcloak (talk) 20:25, 15 September 2012 (UTC)
- The whole section needs working over - not only is it badly written, CO is not the only gas with an indirect radiative effect and the reference used is not the most appropriate for this type of article (a description of a single experiment not a synthesis). There is much more in section 2.10.3 of IPCC 4AR.[8]. I will have a go at doing something better.--NHSavage (talk) 16:51, 2 December 2012 (UTC)
- ^ http://phorums.com.au/archive/index.php?s=1cd2f6d0b1897c4567b59141fa380857&api=1
- ^ Most countries in the world are Parties to the United Nations Framework Convention on Climate Change (UNFCCC), which has adopted the 2 °C target. There are currently (as of November 25, 2011) 195 Parties (194 states and 1 regional economic integration organization (the European Union)) to the UNFCCC. United Nations Framework Convention on Climate Change (2011). "Status of Ratification of the Convention". Retrieved 2011-11-25.
- ^ United Nations Framework Convention on Climate Change (15 March 2011), Conference of the Parties - Sixteenth Session: Decision 1/CP.16: The Cancun Agreements: Outcome of the work of the Ad Hoc Working Group on Long-term Cooperative Action under the Convention (English): Paragraph 4 (PDF), p. 3
- ^ United Nations Environment Programme (UNEP) (November 2011), "Executive Summary", Bridging the Emissions Gap: A UNEP Synthesis Report (PDF), p. 8, ISBN 978-92-807-3229-0 UNEP Stock Number: DEW/1470/NA
- ^ International Energy Agency (2011), "Executive Summary (English)", World Energy Outlook 2011 (PDF), p. 2
