User:Hhhippo/Sandbox
Template drafts for WP:MOSPHYS: sxt, !sxt
Example usage: E = mc2, E = mc2, no title, E = mc2, E = mc2, no title
Atom[edit]
Energy levels[edit]
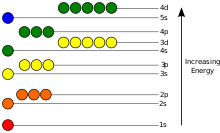
The energy of an electron in an atom is composed of its potential energy, stemming mostly from the electric field generated by the nucleus and the other electrons, and its kinetic energy, resulting from its movement. In the quantum-mechanical model, this total energy can only assume certain values, each corresponding to a particular atomic orbital, a quantum state described by a wave function centered on the nucleus. An electron's energy level can be characterized by the ionization energy, the energy needed to remove the electron from the atom, usually given in units of electronvolts (eV).
Atomic orbitals are described by three quantum numbers: the principal quantum number n, the azimuthal quantum number ℓ and the magnetic quantum number m. Each orbital can host up to two electrons with different spin quantum number s. An atom with an overall electron configuration corresponding to the lowest possible total energy is said to be in its ground state. If one or more electrons occupy a bound state of higher energy than the lowest one available to them, the atom is in an excited state.
Electrons can transition to available states of higher energy if the energy required by this process is provided, for example by absorption of a photon. Similarly, they can transition to available lower energy states while releasing the surplus energy, for example by emission of a photon. Due to the quantized nature of the electron levels, atoms only absorb and emit photons of specific energies and therefore specific frequencies within the electromagnetic spectrum. Each element has a characteristic spectrum of transition energies given by the interplay of its nuclear charge and the possible electron configurations.
[to be continued...][1]

When a continuous spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of emission lines from the photons emitted by the atoms.) Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.[2]
Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin–orbit coupling, which is an interaction between the spin and motion of the outermost electron.[3] When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.[4] The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.[5]
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.[6]
Talk[edit]
Complications to avoid in this summary:
- Potential energy is often, but not always defined to be zero at infinite separation.
- Energy levels describe the total energy, not the potential energy.
- Binding energy can be referred to the vacuum level or the fermi level.
- There's a difference between energy levels of an individual electron and the atom as a whole.
ToDo:
- Reduce overlap with #electron cloud.
References[edit]
- ^ Cite error: The named reference
martin2007was invoked but never defined (see the help page). - ^ Cite error: The named reference
avogadrowas invoked but never defined (see the help page). - ^ Cite error: The named reference
fitzpatrick20070216was invoked but never defined (see the help page). - ^ Cite error: The named reference
weiss2001was invoked but never defined (see the help page). - ^ Beyer 2003, pp. 232–236.
- ^ Cite error: The named reference
watkins_sjsuwas invoked but never defined (see the help page).
