User:Bhendrickson8/sandbox Chemistry2018 Smog
Smog is a type of severe air pollution. The word "smog" was coined in the early 20th century as a blending of the words smoke and fog to refer to smoky fog, its opacity, and odor.[1] The word was then intended to refer to what was sometimes known as pea soup fog, a familiar and serious problem in London from the 19th century to the mid-20th century. This kind of visible air pollution is composed of nitrogen oxides, sulphur oxides, ozone, smoke and other particulates. Man-made smog is derived from coal combustion emissions, vehicular emissions, industrial emissions, forest and agricultural fires and photochemical reactions of these emissions.
Smog is often categorized as being either summer smog or winter smog. Summer smog is primarily associated with the photochemical formation of ozone. During the summer season when the temperatures are warmer and there is more sunlight present, photochemical smog is the dominant type of smog formation. During the winter months when the temperatures are colder, and atmospheric inversions are common, there is an increase in coal and other fossil fuel usage to heat homes and buildings. These combustion emissions, together with the lack of pollutant dispersion under inversions, characterize winter smog formation. While photochemical smog is the main smog formation mechanism during summer months, winter smog episodes are still common. Smog formation in general relies on both primary and secondary pollutants. Primary pollutants are emitted directly from a source, such as emissions of sulfur dioxide from coal combustion. Secondary pollutants, such as ozone, are formed when primary pollutants undergo chemical reactions in the atmosphere.
Photochemical smog, as found for example in Los Angeles, is a type of air pollution derived from vehicular emission from internal combustion engines and industrial fumes. These pollutants react in the atmosphere with sunlight to form secondary pollutants that also combine with the primary emissions to form photochemical smog. In certain other cities, such as Delhi, smog severity is often aggravated by stubble burning in neighboring agricultural areas. The atmospheric pollution levels of Los Angeles, Beijing, Delhi, Lahore, Mexico City, Tehran and other cities are often increased by an inversion that traps pollution close to the ground. The developing smog is usually toxic to humans and can cause severe sickness, or a shortened life span, premature death.
Causes[edit]
Coal[edit]
Coal fires can emit significant clouds of smoke that contribute to the formation of winter smog. Coal fires can be used to heat individual buildings or to provide energy in a power-producing plant. Air pollution from this source has been reported in England since the Middle Ages.[2][3]London, in particular, was notorious up through the mid-20th century for its coal-caused smogs, which were nicknamed 'pea-soupers.' Air pollution of this type is still a problem in areas that generate significant smoke from burning coal. The emissions from coal combustion are one of the main causes of air pollution in China.[4] Especially during autumn and winter when coal-fired heating ramps up, the amount of produced smoke at times forces some Chinese cities to close down roads, schools or airports. One prominent example for this was China's Northeastern city of Harbin in 2013.
Transportation emissions[edit]
Traffic emissions – such as from trucks, buses, and automobiles– also contribute to the formation of smog.[5] Airborne by-products from vehicle exhaust systems cause air pollution and are a major ingredient in the creation of smog in some large cities.[6][7][8][9]
The major culprits from transportation sources are carbon monoxide (CO),[10][11]nitrogen oxides (NO and NOx),[12][13][14]volatile organic compounds,[11][12] and hydrocarbons (hydrocarbons are the main component of petroleum fuels such as gasoline and diesel fuel).[11] Transportation emissions also include sulfur dioxides and particulate matter but in much smaller quantities than the pollutants mentioned previously. The nitrogen oxides and volatile organic compounds can undergo a series of chemical reactions with sunlight, heat, ammonia, moisture, and other compounds to form the noxious vapors, ground level ozone, and particles that comprise smog.[11][12]
Photochemical smog[edit]
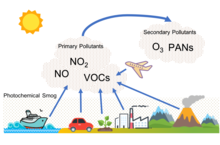
Photochemical smog, often referred to as summer smog, is the chemical reaction of sunlight, nitrogen oxides and volatile organic compounds in the atmosphere, which leaves airborne particles and ground-level ozone.[16] Photochemical smog depends on primary pollutants as well as the formation of secondary pollutants. These primary pollutants include nitrogen oxides, particularly nitric oxide (NO) and nitrogen dioxide (NO2), and volatile organic compounds. The relevant secondary pollutants include peroxylacyl nitrates (PAN), tropospheric ozone, and aldehydes. An important secondary pollutant for photochemical smog is ozone, which is formed when hydrocarbons (HC) and nitrogen oxides (NOx) combine in the presence of sunlight; nitrogen dioxide (NO2), which is formed as nitric oxide (NO) combines with oxygen in the air.[17] In addition, when SO2 and NOx are emitted they eventually are oxidized in the troposphere to nitric acid and sulfuric acid, which, when mixed with water, form the main components of acid rain.[18] All of these harsh chemicals are usually highly reactive and oxidizing. Photochemical smog is therefore considered to be a problem of modern industrialization. It is present in all modern cities, but it is more common in cities with sunny, warm, dry climates and a large number of motor vehicles.[19] Because it travels with the wind, it can affect sparsely populated areas as well.
The composition and chemical reactions involved in photochemical smog were not understood until the 1950s. In 1948, flavor chemist Arie Haagen-Smit adapted some of his equipment to collect chemicals from polluted air, and identified ozone as a component of Los Angeles smog. Haagen-Smit went on to discover that nitrogen oxides from automotive exhausts and gaseous hydrocarbons from cars and oil refineries, exposed to sunlight, were key ingredients in the formation of ozone and photochemical smog.[20]: 219–224 [21][22] Haagen-Smit worked with Arnold Beckman, who developed various equipment for detecting smog, ranging from an "Apparatus for recording gas concentrations in the atmosphere" patented on October 7, 1952, to "air quality monitoring vans" for use by government and industry.[20]: 224–226
Formation and Reactions[edit]
During the morning rush hour, a high concentration of nitric oxide and hydrocarbons are emitted to the atmosphere, mostly via on-road traffic but also from industrial sources. Some hydrocarbons are rapidly oxidized by OH· and form peroxy radicals, which convert nitric oxide (NO) to nitrogen dioxide (NO2).
(1)
(2)
(3)
Nitrogen dioxide (NO2) and nitric oxide (NO) further react with ozone (O3) in a series of chemical reactions:
(4) ,
(5)
(6)
This series of equations is referred to as the photostationary state (PSS). However, because of the presence of Reaction 2 and 3, NOx and ozone are not in a perfect steady state. By replacing Reaction 6 with Reaction 2 and Reaction 3, the O3 molecule is no longer destroyed. Therefore, the concentration of ozone keeps increasing throughout the day. This mechanism can escalate the formation of ozone in smog. Other reactions such as the photooxidation of formaldehyde (HCHO), a common secondary pollutant, can also contribute to the increased concentration of ozone and NO2. Photochemical smog is more prevalent during summer days since incident solar radiation fluxes are high, which favors the formation of ozone (reactions 4 and 5). The presence of a temperature inversion layer is another important factor. That is because it prevents the vertical convective mixing of the air and thus allows the pollutants, including ozone, to accumulate near the ground level, which again favors the formation of photochemical smog.
There are certain reactions that can limit the formation of O3 in smog. The main limiting reaction in polluted areas is:
(7)
This reaction removes NO2 which limits the amount of O3 that can be produced from its photolysis (reaction 4). HNO3 is a sticky compound that can easily be removed onto surfaces (dry deposition) or dissolved in water and be rained out (wet deposition). Both ways are common in the atmosphere and can efficiently remove the radicals and nitrogen dioxide.

Natural causes[edit]
An erupting volcano can emit high levels of sulfur dioxide along with a large quantity of particulate matter; two key components to the creation of smog. However, the smog created as a result of a volcanic eruption is often known as vog to distinguish it as a natural occurrence. The chemical reactions that form smog following a volcanic eruption are different than the reactions that form photochemical smog. The term smog encompasses the effect when a large amount of gas phase molecules and particulate matter are emitted to the atmosphere, creating a visible haze. The event causing a large amount of emissions can vary but still result in the formation of smog.
Plants are another natural source of hydrocarbons that could undergo reactions in the atmosphere and produce smog. Globally both plants and soil contribute a substantial amount to the production of hydrocarbons, mainly by producing isoprene and terpenes.[23] Hydrocarbons released by plants can often be more reactive than man-made hydrocarbons. For example when plants release isoprene, the isoprene reacts very quickly in the atmosphere with hydroxyl radicals. These reactions produce hydroperoxides which increase ozone formation.[24]
- ^ Schwartz Cowan, Ruth (1997). A Social History of American Technology. Oxford University Press. ISBN 978-0-19-504605-2.[page needed]
- ^ Chris (2007). "Environmentalism in 1306". By Environmental Graffiti. Archived from the original on 2008-07-25.
- ^ Karl (2008). "Environmentalism in 1306". By Environmental Graffiti.
- ^ Heilmann, Sebastian (ed.) (2017). China's Political System. Rowman & Littlefield. p. 360. Archived from the original on 2017-05-06.
{{cite book}}:|first=has generic name (help) - ^ "Clearing the Air". The Surface Transportation Policy Project. 19 August 2003. Archived from the original on 8 February 2007. Retrieved 2007-04-26.
- ^ "EPA Tools Available as Summer Smog Season Starts" (Press release). Boston, Massachusetts: United States Environmental Protection Agency. 30 April 2008.
- ^ "Sprawl Report 2001: Measuring Vehicle Contribution to Smog". Sierra Club. 2001.
- ^ "Smog – Causes". The Environment: A Global Challenge. Retrieved 25 October 2013.
- ^ "Smog — Who Does It Hurt? What You Need to Know About Ozone and Your Health (EPA-452/K-99-001)" (PDF). United States Environmental Protection Agency. July 1999.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "State and County Emission Summaries: Carbon Monoxide". Air Emission Sources. United States Environmental Protection Agency. 25 October 2013.
- ^ a b c d "Motor vehicle pollution". Queensland Government. 4 April 2013.
- ^ a b c "Health". Nitrogen Dioxide. United States Environmental Protection Agency. 14 February 2013.
- ^ "The Regional Transport of Ozone: New EPA Rulemaking on Nitrogen Oxide Emissions (EPA-456/F-98-006)" (PDF). United States Environmental Protection Agency. September 1998.
- ^ "State and County Emission Summaries: Nitrogen Oxides". Air emission sources. United States Environmental Protection Agency. 25 October 2013.
- ^ "ESS Topic 6.3: Photochemical Smog". AMAZING WORLD OF SCIENCE WITH MR. GREEN. Retrieved 2018-11-15.
- ^ "Nox/VOC Smog Fact Sheet" (PDF). Canadian Council of Ministers of the Environment. Archived from the original (PDF) on 2011-09-28.
{{cite journal}}: Cite journal requires|journal=(help) - ^ http://www.pmfias.com/2015/08/Smog-Photochemical-smog.html[permanent dead link]
- ^ "Acid Rain Students Site: What causes acid rain?". www3.epa.gov. Retrieved 2018-11-05.
- ^ Miller, Jr., George Tyler (2002). Living in the Environment: Principles, Connections, and Solutions (12th ed.). Belmont: The Thomson Corporation. p. 423. ISBN 0-534-37697-5.
- ^ a b Thackray, Arnold & Myers, Jr., Minor (2000). Arnold O. Beckman : one hundred years of excellence. Philadelphia, Pa.: Chemical Heritage Foundation. ISBN 978-0-941901-23-9.
- ^ Gardner, Sarah (14 July 2014). "LA Smog: the battle against air pollution". Marketplace.org. American Public Media. Retrieved 6 November 2015.
- ^ Kean, Sam (2015). "The Flavor of Smog". Distillations. 2 (3): 5. Retrieved 22 March 2018.
- ^ Elsevier. "Chemistry of the Natural Atmosphere, Volume 71 - 2nd Edition". www.elsevier.com. Retrieved 2018-11-15.
- ^ Sharkey, T. D.; Wiberley, A. E.; Donohue, A. R. (2007-10-17). "Isoprene Emission from Plants: Why and How". Annals of Botany. 101 (1): 5–18. doi:10.1093/aob/mcm240. ISSN 0305-7364. PMC 2701830. PMID 17921528.








