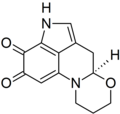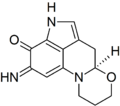Wikipedia talk:WikiProject Chemicals/Archive 2010
Archived discussion of WT:CHEMS in 2010
Merge oxalate and oxalic acid at Talk:oxalic acid[edit]
An determined and now slightly bitter editor User:Yappy2bhere has proposed the merger of oxalate and oxalic acid. I removed the tags, explaining that we have "much precedent for separate articles acids and conjugate bases and elements and their redox partners" But the editor is determined to see this merger discussion play out, so experienced editors are encouraged to communicate at Talk:oxalic acid on the advisability of the merger. The we need to write this down in our MOS.--Smokefoot (talk) 14:53, 16 January 2010 (UTC)
WP 1.0 bot announcement[edit]
This message is being sent to each WikiProject that participates in the WP 1.0 assessment system. On Saturday, January 23, 2010, the WP 1.0 bot will be upgraded. Your project does not need to take any action, but the appearance of your project's summary table will change. The upgrade will make many new, optional features available to all WikiProjects. Additional information is available at the WP 1.0 project homepage. — Carl (CBM · talk) 03:06, 22 January 2010 (UTC)
Deletion discussion[edit]
Two chemical articles, claimed to be hoaxes, are up for deletion at Wikipedia:Articles for deletion/ICA (4-Icaroldichloridesulphate aka Icarus). Input from additional chemists would be helpful. -- Ed (Edgar181) 15:02, 28 January 2010 (UTC)
New nuclear chemicals[edit]
I found a few new articles about chemicals related to the nuclear industry, I will have a look later, but helping hands are welcome. Plutonium-gallium alloy, Uranium hydride, AlSiC, Barium borate and Dymalloy. Thanks. --Stone (talk) 11:46, 7 February 2010 (UTC)
List of highly toxic gases = OR and should be deleted?[edit]
I tagged this article as being original research. Advice welcome because possibly I am missing something
- who decided that these gases are toxic?
- who decided that these gases represent a problem. H2Te is highly unstable at room temperature, and how often does one encounter dichloroacetylene?
- who decides on what qualifies as a gas? hexachlorobutadiene is listed (boiling point = 210 °C)
Comments welcome.--Smokefoot (talk) 17:07, 7 February 2010 (UTC)
- May also show a non-worldwide view - all the data is US, not sure how well EU limits and other countries limits would compare. Bromine is also a liquid, it is therefore going to add all low boiling liquids to it? What criteria decides when a "gas" is toxic enough to be included? May also give a false impression if some things are omitted - e.g It's not in the list so it's not that bad - just pass me the dimethyl sulphate... Ronhjones (Talk) 01:45, 25 February 2010 (UTC)
Lanthanide, actinide vs. lanthanoid, actinoid[edit]
IUPAC uses the latter and some editors have been changing everything to that standard. However a little searching tells me that actual adoption of the -oid names is low. --JWB (talk) 23:24, 9 February 2010 (UTC)
- Just for a start, a mass changeover without discussion here is certainly unhelpful. Please feel free to invite the editors who are trying to the mass changeover to discuss things here first. The question of how far we follow (or not) IUPAC nomenclature has often been a source of contention (in both directions), and has even been the subject of comment (in both directions) from senior members of the IUPAC committees concerned! But let's just keep the debate to the subject in question: do we say "lanthanide", do we say "lanthanoid", or do we say both (one or the other depending on the author)? Physchim62 (talk) 02:55, 10 February 2010 (UTC)
- Lanthanide, mainly because that term is most commonly used by chemists and materials people (and partly to resist the nomenclature police).--Smokefoot (talk) 03:25, 10 February 2010 (UTC)
- I would second that - lanthanoid is rarely used, no matter how official it is, and we know many examples of official terms which have not been adopted by scientists. Materialscientist (talk) 03:30, 10 February 2010 (UTC)
- There has been dicussion about moving the pages on the Actinoid and lanthanoid talk pages, these moves were completed literally years ago and there has been plenty of time for discussion. All I have done recently is change the wikilinks from "what links here" regarding the pages Actinide and Lanthanide that were moved some time ago, which is standard procedure following page moves I believe but wasn't done at the time. I was acting in good faith and was simply clearing up two old page moves. I certainly wasn't trying to pull the wool over anybody's eyes and reject that I was trying to make a mass changeover without discussion. Admittedely I did also move the page Lanthanoid contraction but one page doesn't consitute a mass changeover. I take full responsibility for my edits and if IUPAC convention is rejected then I will of course undo all of my actions. I favour the IUPAC convention and have seen it used. Although I think it is fair to say that academic journals do not appear to have updated their own MOS in keeping with the change. The wikipedia periodic table currently uses the IUPAC convention as does the webelements website. Jdrewitt (talk) 07:21, 10 February 2010 (UTC)
- I myself would also use 'lanthanide' for these terms, and if I look through the comments in this thread, it looks like that is the common way around (small pool, but IIRC I see an American, some people in Europe and an Australian (???) editor commenting. We have here in chemistry generally said 'use the name that is commonly used', not the official one (exceptions when there is multiple use of the same term (XTC, MDMA), etc. etc.), and let redirects do the work for those who do search for the IUPAC term.
- Don't worry, Jdrewitt, lets discuss and see where it goes. Bold changes can indeed be undone, no harm done. --Dirk Beetstra T C 08:12, 10 February 2010 (UTC)
I appreciate the good faith efforts to update to what is billed as the most modern terminology, but the difference in search counts was so great it surprised me, for example Google Scholar lanthanide: 165,000, lanthanoid: 6,680, actinide: 78,700, actinoid: 1,640. --JWB (talk) 11:45, 10 February 2010 (UTC)
- How does this comparison look when you compare articles published since the IUPAC nomenclature change? I'm not sure what year this was although I think it was fairly recent perhaps 2005? Jdrewitt (talk) 11:51, 10 February 2010 (UTC)
- The -oid endings have been around since at least the 1990 Recommendations, although the wording was made stronger ("lanthanoid and actinoid are preferred to lanthanide and actinide") in the 2005 Recommendations. Physchim62 (talk) 12:00, 10 February 2010 (UTC)
- I checked, google scholar reports reports 15,400 hits for Lanthanide and 1710 for Lanthanoid for articles published since 2005. Hence Lanthanoid is used 10% of the time. This is an increase from 4% of the share from the figures stated above which presumably includes all years. So although -oid may not be the most common term it does appear to be increasing in usage. There doesn't appear to be a change in the usage of actinide yet. Jdrewitt (talk) 12:05, 10 February 2010 (UTC)
- A check of lanthanide vs lanthanoid in titles in the American Chem. Soc. journal Inorganic Chemistry: lanthanide: 570 vs 24 for lanthanoid. --Smokefoot (talk) 14:04, 10 February 2010 (UTC)
- For what years? If the use of -oid is becoming more commonplace then it will be more abundant in recent articles vs. older onse, as google scholar seems to show (which covers all journals). Jdrewitt (talk) 14:09, 10 February 2010 (UTC)
- Inorg Chem has been published since 1963. Lanthanoid first appeared in 2 titles in 1980, it was used once in 2010. Lanthanide was used in 8 titles in 2010. I didnt check but I assume that it has been used throughout the 48 years of this journal. --Smokefoot (talk) 14:30, 10 February 2010 (UTC)
- Ok so that'll be 11% then in 2010! My point though was that although -oid is not the most common terminology, its use does appear to be increasing. I'm not certain whether academics are intentionally ignoring IUPAC convention or just unaware of it. Jdrewitt (talk) 14:56, 10 February 2010 (UTC)
- I publish lanthanide and actinide, and it is significantly more common in the biased subset of papers on my computer. I vaguely prefer -oid for here; unless and until it actually becomes more common, I would be fine with asserting WP:COMMONNAME to go back to -ide. Please let me know if you would like me to move actinoid, as I am not sure if the software counts the move vandalism here as non-trivial history. - 2/0 (cont.) 17:22, 10 February 2010 (UTC)
- Ok so that'll be 11% then in 2010! My point though was that although -oid is not the most common terminology, its use does appear to be increasing. I'm not certain whether academics are intentionally ignoring IUPAC convention or just unaware of it. Jdrewitt (talk) 14:56, 10 February 2010 (UTC)
- Inorg Chem has been published since 1963. Lanthanoid first appeared in 2 titles in 1980, it was used once in 2010. Lanthanide was used in 8 titles in 2010. I didnt check but I assume that it has been used throughout the 48 years of this journal. --Smokefoot (talk) 14:30, 10 February 2010 (UTC)
- For what years? If the use of -oid is becoming more commonplace then it will be more abundant in recent articles vs. older onse, as google scholar seems to show (which covers all journals). Jdrewitt (talk) 14:09, 10 February 2010 (UTC)
- A check of lanthanide vs lanthanoid in titles in the American Chem. Soc. journal Inorganic Chemistry: lanthanide: 570 vs 24 for lanthanoid. --Smokefoot (talk) 14:04, 10 February 2010 (UTC)
- I checked, google scholar reports reports 15,400 hits for Lanthanide and 1710 for Lanthanoid for articles published since 2005. Hence Lanthanoid is used 10% of the time. This is an increase from 4% of the share from the figures stated above which presumably includes all years. So although -oid may not be the most common term it does appear to be increasing in usage. There doesn't appear to be a change in the usage of actinide yet. Jdrewitt (talk) 12:05, 10 February 2010 (UTC)
- The -oid endings have been around since at least the 1990 Recommendations, although the wording was made stronger ("lanthanoid and actinoid are preferred to lanthanide and actinide") in the 2005 Recommendations. Physchim62 (talk) 12:00, 10 February 2010 (UTC)
- The discussion above points to lanthanide over lanthanoid and, by inference, actinide over actinoid. When the -oid naming might become dominant, which might not be soon, we should revisit the issue. --Smokefoot (talk) 18:24, 10 February 2010 (UTC)
- It's not quite as simple as simply moving back. There are articles such as Periodic table which use the IUPAC nomenclature and I'm sure the people there would like to make some input first. Changing the article titles would also mean universally changing all references of -oid. I'm not saying this shouldn't be done, just that these things need to be considered first. The articles were moved several years back, I don't see any need for haste. Also, if the moves are made what do we say in the lead, we have to acknowledge the IUPAC nomenclature. Instead of writing Lanthanoid (formally lanthanide) should we write something like Lanthanide (also lanthanoid per IUPAC nomenclature)? Jdrewitt (talk) 18:45, 10 February 2010 (UTC)
- Yes, inevitably there will be lots of clean-up and possibly some automated method could be devised to search and replace the oid, assuming that the consensus continues to flow in the current direction. It is difficult to understand how this obscure term ever got inserted so often, but it's too late to complain. Per our tradition (but apparently not in our Chem manual of style, in cases where the IUPAC name differs from the commonly used name, our practice has been to put the IUPAC name in parenthesis adjacent to the common name in the lead sentence, but otherwise not encumber the article with two names.--Smokefoot (talk) 18:58, 10 February 2010 (UTC)
- I think this should be made clearer in the policy. I think it only requires a sentence or two that goes something like "in the event that common usage of a chemical term differs from IUPAC nomenclature then ...." you get the idea. Ok, I think there is overwhelming evidence that despite the IUPAC convention -ide is the common usage term so putting personal preferences aside wikipedia should use the (overwhelmingly) most common term. I will undo my recent actions with regards to the cleanup I was performing from the original page moves!! Jdrewitt (talk) 19:15, 10 February 2010 (UTC)
- Yes, inevitably there will be lots of clean-up and possibly some automated method could be devised to search and replace the oid, assuming that the consensus continues to flow in the current direction. It is difficult to understand how this obscure term ever got inserted so often, but it's too late to complain. Per our tradition (but apparently not in our Chem manual of style, in cases where the IUPAC name differs from the commonly used name, our practice has been to put the IUPAC name in parenthesis adjacent to the common name in the lead sentence, but otherwise not encumber the article with two names.--Smokefoot (talk) 18:58, 10 February 2010 (UTC)
- It's not quite as simple as simply moving back. There are articles such as Periodic table which use the IUPAC nomenclature and I'm sure the people there would like to make some input first. Changing the article titles would also mean universally changing all references of -oid. I'm not saying this shouldn't be done, just that these things need to be considered first. The articles were moved several years back, I don't see any need for haste. Also, if the moves are made what do we say in the lead, we have to acknowledge the IUPAC nomenclature. Instead of writing Lanthanoid (formally lanthanide) should we write something like Lanthanide (also lanthanoid per IUPAC nomenclature)? Jdrewitt (talk) 18:45, 10 February 2010 (UTC)
- While I do see the merit in the COMMONNAME argument, I am a fan of following IUPAC conventions where possible. The reason for the naming is relevant; I believe it was because the former terms ending -ide resembled compound names like chloride, oxide and so were felt to be confusing for learners. I certainly don't think anybody should be doing mass edits until a firm and wide consensus is established here. --John (talk) 20:04, 10 February 2010 (UTC)
- Well I have undone the 146 AWB edits that I made yesterday - I should have got consensus before I made them in the first place but I didn't think they would be controversial bearing in mind how old the original page moves were. I'm putting this one down to experience. It didn't take too long...
- If the decision is made that the IUPAC nomenclature should be used then we can get a bot to do all the donkey work instead and then be sure that the whole of wikipedia is IUPAC ok! :) But I think it will be unlikely that consensus will be reached in favour of IUPAC nomenclature bearing in mind the overwhelmingly greater use of -ide in the scientific community. Jdrewitt (talk) 20:14, 10 February 2010 (UTC)
- The naming of articles with two valid names is always a problem. I saw that the strict use of common usage names is as problematic as the strict sticking to IUPAC, which is a organization which has no ruling power over chemistry or even over Wikipedia. Be Bold, but not stupid is the only suggestion I have for the problem. The fights about the use of sulfur/sulphur and aluminium/aluminum are legend in the WPElements, and I am not sure if we always do the right thing. The German wiki has an even better built in stupidity for non German words. They have a book somewhere with a list of how often a word is used and if this number is below X you have to use the foreign writing and if it is above it is by definition a German word and you are allowed to use the German writing. There was the discussion about a small town in the east and the article with the German name was renamed after the usage dropped below the threshold. So a edit war and a long lasting drama which wastes time was the result.--Stone (talk) 21:40, 10 February 2010 (UTC)
- I think sulfur/sulphur and aluminium/aluminum are British vs. American spelling, for which there is a general Wikipedia policy independent of chemistry. --JWB (talk) 21:49, 10 February 2010 (UTC)
- Sorry, but there the project says that IUPAC is above British vs. American spelling policies. This is problematic and always gives long discussions. A article started in british English about Aluminium sulphate and an american one about Aluminum sufite would be perfect with the British vs. American spelling policies, but would make the project as a whole difficult to maintain.--Stone (talk) 21:56, 10 February 2010 (UTC)
- I think sulfur/sulphur and aluminium/aluminum are British vs. American spelling, for which there is a general Wikipedia policy independent of chemistry. --JWB (talk) 21:49, 10 February 2010 (UTC)
- The naming of articles with two valid names is always a problem. I saw that the strict use of common usage names is as problematic as the strict sticking to IUPAC, which is a organization which has no ruling power over chemistry or even over Wikipedia. Be Bold, but not stupid is the only suggestion I have for the problem. The fights about the use of sulfur/sulphur and aluminium/aluminum are legend in the WPElements, and I am not sure if we always do the right thing. The German wiki has an even better built in stupidity for non German words. They have a book somewhere with a list of how often a word is used and if this number is below X you have to use the foreign writing and if it is above it is by definition a German word and you are allowed to use the German writing. There was the discussion about a small town in the east and the article with the German name was renamed after the usage dropped below the threshold. So a edit war and a long lasting drama which wastes time was the result.--Stone (talk) 21:40, 10 February 2010 (UTC)
I would urge the project to be practical and make a choice. Jdrewitt's long-spamming my watchlist twice yesterday was a call for that :-) I think we are seeking some rationale, a justification which doesn't exist, and we should just set a rule and follow it. It is quite same as most other our conventions, which half of the external editors will not understand anyway until we just say "this is a convention chosen on WP". Following IUPAC everywhere is one choice, but they did adopt names for some chemicals which are extremely unpopular everywhere. The argument that -ide sounds like a compound is also valid. On the other side, 10% stats is much too low and can beat any logic. I'm starting a vote below (support/oppose). Please remove or ignore it, but if vote, please be brief. Materialscientist (talk) 23:37, 10 February 2010 (UTC)
- Vagina, a recommended medical term, gets 21 million hits whereas pussy, a commonly used slang term, gets 64 million hits. Should we apply WP:COMMONNAME and move vagina to pussy? Of course not! We should abide by the recommendations put forth by the medical governing body! Does anyone see the obvious parallel? --Cryptic C62 · Talk 03:31, 12 February 2010 (UTC)
- Nope, and you can consider removing the above comment for several obvious reasons, including multiple meaning of "pussy" and comparison of vastly different terms and fields. Materialscientist (talk) 03:41, 12 February 2010 (UTC)
- A perfect example of why a Google test should be used with much care. --Dirk Beetstra T C 07:05, 12 February 2010 (UTC)
- I see no parallel, besides we didn't perform a google search we used google scholar which only searches academic texts. And we get the same results if we consult academic journals directly and search for the same terms. It's fine to use google provided you must also apply common sense! Jdrewitt (talk) 08:52, 12 February 2010 (UTC)
- Just to be sure, Google Scholar gives 253,000 hits for 'vagina' and only 28,200 hits for 'pussy' (where the first 10 hits do not all use the term 'pussy' as a synonym of 'vagina'). I hope this helps. --Dirk Beetstra T C 09:22, 12 February 2010 (UTC)
- My argument was not meant to be the ultimate refutation of all that has been said (nor was it meant to offend), it was just meant to demonstrate that the obviously correct term won't always be used more than the vernacular term. For those of you who thought my example was too unrelated, non-Google-scholary, or vulgar to be taken into consideration, have at ye: caesium receives ~46k scholarly hits, cesium receives ~315k scholarly hits. This also puts the "10%" argument in perspective since caesium is only used 15% of the time. --Cryptic C62 · Talk 16:13, 12 February 2010 (UTC)
- I don't see how this changes the perspective. We were looking at which term was in predominant usage and it is clear from consulting both google scholar and individual journals that -ide is the most predominent term. All you have shown is that cesium is the most predominent term used over caesium. Are you suggesting that this isn't actually the case? Jdrewitt (talk) 17:30, 12 February 2010 (UTC)
- Allow me to clarify. Although "cesium" gets more Google scholar hits, "caesium" is the IUPAC recommended name and "caesium" is our article. The current logic being put forth in this argument is that because "lanthanide" is more commonly used than "lanthanoid", "lanthanide" should be our article even though "lanthanoid" is recommended by IUPAC. These two contradict each other. We shouldn't abide by IUPAC for caesium and COMMONNAME for lanthanide; we should be consistent. --Cryptic C62 · Talk 21:27, 12 February 2010 (UTC)
- I would love to have a similar but separate discussion and vote on cæsium after this. I also feel "caesium" is incongruous with most existing literature, although it looks like the citation ratio is not quite as overwhelming. But if the project members, who appear to include a good deal of expertise, feel that IUPAC weighs more than that citation ratio, that's fine with me. --JWB (talk) 23:32, 12 February 2010 (UTC)
- The preference in WP for cæsium (or caesium) vs cesium surprised me too initially, but at least they two spellings are pronounced identically. One of many related issues is the aluminium thing, which is about English-English vs American-English, not IUPAC vs common usage.--Smokefoot (talk) 00:48, 13 February 2010 (UTC)
- Thank you for clarifying Cryptic C62. I see your point. However, earlier in this discussion it was mentioned there were numerous cases where wikipedia does not follow IUPAC convention. Can someone provide examples of these? And I think Smokefoot is correct, in the case of Caesium it is more about spelling convention than a name change. Jdrewitt (talk) 08:27, 13 February 2010 (UTC)
- You can check yourself for the adherence to IUPAC. Historically, naming angst originate from suggestions from drive-by editors that contribute little serious content and are therefore perceived as being well-intentioned but professionally naive.--Smokefoot (talk) 14:04, 13 February 2010 (UTC)
- Sorry I wasn't asking anyone to do any unecessary hard work, I just thought since some of you have been involved with these sort of IUPAC issues for some time you may have some really obvious examples. I've looked through the archives, there are of course numerous discussions about the use of IUPAC nomenclature, a selection of what I perceive to be some of the more significant/relevant discussions are here: [1], [2], [3], [4]. Jdrewitt (talk) 15:49, 13 February 2010 (UTC)
- You can check yourself for the adherence to IUPAC. Historically, naming angst originate from suggestions from drive-by editors that contribute little serious content and are therefore perceived as being well-intentioned but professionally naive.--Smokefoot (talk) 14:04, 13 February 2010 (UTC)
- Thank you for clarifying Cryptic C62. I see your point. However, earlier in this discussion it was mentioned there were numerous cases where wikipedia does not follow IUPAC convention. Can someone provide examples of these? And I think Smokefoot is correct, in the case of Caesium it is more about spelling convention than a name change. Jdrewitt (talk) 08:27, 13 February 2010 (UTC)
- The preference in WP for cæsium (or caesium) vs cesium surprised me too initially, but at least they two spellings are pronounced identically. One of many related issues is the aluminium thing, which is about English-English vs American-English, not IUPAC vs common usage.--Smokefoot (talk) 00:48, 13 February 2010 (UTC)
- I would love to have a similar but separate discussion and vote on cæsium after this. I also feel "caesium" is incongruous with most existing literature, although it looks like the citation ratio is not quite as overwhelming. But if the project members, who appear to include a good deal of expertise, feel that IUPAC weighs more than that citation ratio, that's fine with me. --JWB (talk) 23:32, 12 February 2010 (UTC)
- Allow me to clarify. Although "cesium" gets more Google scholar hits, "caesium" is the IUPAC recommended name and "caesium" is our article. The current logic being put forth in this argument is that because "lanthanide" is more commonly used than "lanthanoid", "lanthanide" should be our article even though "lanthanoid" is recommended by IUPAC. These two contradict each other. We shouldn't abide by IUPAC for caesium and COMMONNAME for lanthanide; we should be consistent. --Cryptic C62 · Talk 21:27, 12 February 2010 (UTC)
- I don't see how this changes the perspective. We were looking at which term was in predominant usage and it is clear from consulting both google scholar and individual journals that -ide is the most predominent term. All you have shown is that cesium is the most predominent term used over caesium. Are you suggesting that this isn't actually the case? Jdrewitt (talk) 17:30, 12 February 2010 (UTC)
- My argument was not meant to be the ultimate refutation of all that has been said (nor was it meant to offend), it was just meant to demonstrate that the obviously correct term won't always be used more than the vernacular term. For those of you who thought my example was too unrelated, non-Google-scholary, or vulgar to be taken into consideration, have at ye: caesium receives ~46k scholarly hits, cesium receives ~315k scholarly hits. This also puts the "10%" argument in perspective since caesium is only used 15% of the time. --Cryptic C62 · Talk 16:13, 12 February 2010 (UTC)
A vote. A lanthanide(actinide) should be chosen over lanthanoid(actinoid)[edit]
- ide --JWB (talk) 23:50, 10 February 2010 (UTC)
- Support ide -- It doesn't matter how official the term, 10% is far too low, WP:common usage should prevail. Jdrewitt (talk) 07:24, 11 February 2010 (UTC)
- ide, seems for now the one that is commonly used. --Dirk Beetstra T C 08:33, 11 February 2010 (UTC)
- ide, only because of current stats. Materialscientist (talk) 09:41, 11 February 2010 (UTC)
- ide. Should ide prevail, we might edit our MOS to explain this and similar policy implications.--Smokefoot (talk) 14:35, 11 February 2010 (UTC)
- To me it makes most sense to stick with -ide because of its common use. -- Ed (Edgar181) 14:59, 11 February 2010 (UTC)
- ide in general, but allow occasional/appropriate use of "oid" if the need arises. Check consensus with WP:ELEMENTS. Walkerma (talk) 17:18, 11 February 2010 (UTC)
- ide-- we don't need to mindlessly follow IUPAC recommendations, especially alternatives are in much more common parlance. Yilloslime TC 17:08, 12 February 2010 (UTC)
- ide --Itub (talk) 03:08, 16 February 2010 (UTC)
- ide -- I think it will take many years, if ever, that "oid" becomes the well known name Ronhjones (Talk) 21:17, 17 March 2010 (UTC)
Why in God is there a vote?[edit]
IUPAC recommends -oid so it should be -oid. No discussion should be needed. Raistuumum (talk) 03:42, 23 February 2010 (UTC)
- Neither god nor iupac set the style here. It's determined by wiki user consensus. Please respect that. Vsmith (talk) 04:10, 23 February 2010 (UTC)
Moving actinoid[edit]
Apparently, only Lanthanoid was moved. Could an admin move Actinoid? Headbomb {talk / contribs / physics / books} 19:00, 17 March 2010 (UTC)
- Best wait for consensus - there are plenty of admins looking at this talk. Also it might be advisable once we have a good consensus to move protect the pages Ronhjones (Talk) 21:18, 17 March 2010 (UTC)
Phosphine vs phosphane[edit]
I am not sure why PH3 should be -ine when all the PR3 are already called -ines. Why is that? Nergaal (talk) 04:03, 12 February 2010 (UTC)
- This is similar to the discussion above about lanthanides/lanthanoids. Both phosphine and phosphane are IUPAC names, where 'phosphane' is the IUPAC recommended name. However, most people use 'phosphine' to describe PHxR3-x species. It is a matter of WP:COMMONNAME. I hope this explains. --Dirk Beetstra T C 07:07, 12 February 2010 (UTC)
- The discussion highlights a more difficult problem, i.e. that diphosphine refers to an obscure gas (P2H4_ and to a wildly popular class of ligands. I would guess that 99+% viewers of diphosphine really want to read about the organophosphorus ligands, not the gas. A similar problem with phosphine exists, see Phosphines#Phosphine ligands. We need to solve this glitch.--Smokefoot (talk) 00:48, 13 February 2010 (UTC)
- It would have been nicer, IMHO, if IUPAC had never put their boot into this one in the first place! Why should we say not say "phosphine", by analogy to "amine"? A quick search for "niccolate" on Google indicates that scholarly journals are not too bothered about this sort of obvious nomenclature change (it has been "nickelate" since 1976). A search on Google Scholar will find at least one member of the IUPAC Commission on Inorganic Nomenclature (as it was then) publishing papers entitled in contrary the Commission's recommendations. If the very people who write these rules cannot be bothered to enforce them in their own research groups, I feel that they have little or no value. As for "phosphane", it appears that the chemical community has spoken, at least for the time being. Physchim62 (talk) 15:58, 13 February 2010 (UTC)
- The discussion highlights a more difficult problem, i.e. that diphosphine refers to an obscure gas (P2H4_ and to a wildly popular class of ligands. I would guess that 99+% viewers of diphosphine really want to read about the organophosphorus ligands, not the gas. A similar problem with phosphine exists, see Phosphines#Phosphine ligands. We need to solve this glitch.--Smokefoot (talk) 00:48, 13 February 2010 (UTC)
- Physchim62 .. bad boy .. of course you are referring to 'azane' in analogy to 'phosphane' .. --Dirk Beetstra T C 13:44, 22 February 2010 (UTC)
- Beetstra, go on, just try ordering 100 ml of triethylazane and see what the stores manager says to you! I'll warn you, you might need to order a few hundred mils of a ≈5% v/v solution of ethyloxidane in oxidane to recover from his or her reaction! Physchim62 (talk) 14:10, 22 February 2010 (UTC)
- one that's been bugging me is iron(III) chloride
- anyone thoughts on this? —Preceding unsigned comment added by 170.170.59.133 (talk) 18:48, 10 March 2010 (UTC)
ENGVAR / solubility units.[edit]
I am opening a discussion here, since I think this needs a bit of discussion. 12.31.4.5 (talk · contribs) is changing two things on a couple of pages about chemicals, and with both I disagree:
- In the {{Chembox}} they changes 'colourless' to 'colorless'. In my opinion this goes against WP:ENGVAR, though they argues that all references and external links use the American English spelling.
- Also in the {{Chembox}}, they changes '0.015 g / 100 mL (20 °C)' to '0.015 g/100 g (20 °C)' (later to '0.015 g/100 g Water (20 °C)'). If I am correct, 0.015 g / 100 mL means that there is 15 mg of (in this case, ethylbenzene) dissolved in 100 mL total volume of solution, with the solvent being water. I presume here, that the reference that the value is taken from says it as '0.015 g / 100 mL (20 °C)', and hence that is what we report, but there is not a direct reference for this point of data). Though the density of this solution (of ethylbenzene) will be close to 1, that is not the same as 0.015 g / 100 g. They argues that it is 0.015 g ethylbenzene dissolved in 100 mL water, and hence, that 0.015 g / 100 mL is the same as 0.015 g / 100 g (ignoring the small deviation of the density of water, which is not '1.000' at 20 °C, but I can live with that small deviation).
The second point does brings a next question. IMHO, it would be good to have all values in the same unit. Solubility_table (as 12.31.4.5 argues) is in g / 100 g, and it would be nice to have all in the same unit (we might even consider to use an alternative parameter (e.g. Solubility_standard, like can be done with 'BoilingPt' -> 'BoilingPtC'), which would standardize our information a bit further (I did have a glance at WP:UF, though there is not a standard chemitry microformat ..).
Basically, we disagree on these points, but I think it is useful to discuss this in a wider community. Thanks. --Dirk Beetstra T C 08:32, 18 February 2010 (UTC)
- I've also crossed with some anon who claimed that g/100g is a standard on WP; I just reverted them because it is not - most WP articles are in g/100 mL. The solubility table is for water, and given the accuracy of those values, it is safe to change, IMHO, g/100g to g/100 mL, but. I was wondering why listing values in g/100 mL and not in g/L ? I don't see any problem with ENGVAR - the policy is quite clear on which spelling to keep. Materialscientist (talk) 08:46, 18 February 2010 (UTC)
- For 0.015 g / 100 g to 0.015 g / 100 mL, yes .. but if we are talking brine, I don't think that 26.4 g / 100g is 26.4 g / 100 mL there .. --Dirk Beetstra T C 08:50, 18 February 2010 (UTC)
I have conducted a reassessment of the above article as part of the GA Sweeps process. You are being notified as this project's banner is on the talk page. I have found some concerns which you can see at Talk:Vitamin C/GA1. I have placed the article on hold whilst these are fixed. Thanks. Jezhotwells (talk) 23:08, 19 February 2010 (UTC)
WP:ELEMENTS started creating books on each individual elements. Since there are a lot of them, any help would be very much appreciated. Headbomb {ταλκκοντριβς – WP Physics} 02:32, 28 February 2010 (UTC)
Hi guys
Can you guys do a sanity check of this image:
Before I put it up on the article? Thanks. --Rifleman 82 (talk) 13:40, 1 March 2010 (UTC)
- The addition of a Grignard reagent to a nitrile won't normally provide a methyl group. Either something is wrong in the image, or some very unusual chemistry is going on there. -- Ed (Edgar181) 14:44, 1 March 2010 (UTC)
Sorry, the paper (Hans Bock, Ilka Goebel, Zdenek Havlas, Siegfried Liedle, Heinz Oberhammer (1991). "Triisopropylamine: A Sterically Overcrowded Molecule with a Flattened NC3 Pyramid and a "p-Type" Nitrogen Electron Pair". Angew. Chem. Int. Ed. 30 (2): 187–190. doi:10.1002/anie.199101871.{{cite journal}}: CS1 maint: multiple names: authors list (link)) said CH3HgCl, not the Grignard. I suppose it was relatively unusual that I typed Mg in place of Hg. That aside, does it make sense? --Rifleman 82 (talk) 14:49, 1 March 2010 (UTC)
- Actually, now that I think about it a moment, the reaction will proceed through elimination of HCN, followed by addition of the methylmercury to the resulting imine. So, yes, this makes sense to me now. -- Ed (Edgar181) 14:59, 1 March 2010 (UTC)
- Ah, that's great. Thanks. I'm not familiar with mercury used this way. --Rifleman 82 (talk) 15:03, 1 March 2010 (UTC)
Hi,
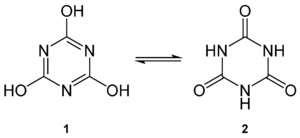
Please, Excuse my english. In this article, it is question of 1,3,5-triaza-cyclohexane-2,4,6-trione (C6N3O3) which seems very strange. I already asked the French chemistry project here and for now, we think it should be 1,3,5-triazinane-2,4,6-trione C3H3N3O3, the tautomer of cyanuric acid which is still very different. The page 97 of the book referenced on the article isn't visible on googlebooks. We found about 1,3,5-triaza-cyclohexane-2,4,6-trione, the reference : Comments on inorganic chemistry, Volume 16, Number 6, 1994, page 104. One of you who have access to this journal in a good science library, could check what is the compound with this name and what is really his formula, please ? --tpa2067 (Allô...) 15:51, 4 March 2010 (UTC) —Preceding unsigned comment added by Tpa2067 (talk • contribs)
- If you type either of these names into ChemDraw, you get the same molecule, tautomer number 2 below (from cyanuric acid).
Ferrous sulfide = sulfanylideneiron?[edit]
PubChem lists the IUPAC name of ferrous sulfide as sulfanylideneiron. Is that correct? Almost nobody seems to use that term. But if it is correct we should definitely add it to our article. AxelBoldt (talk) 23:36, 12 March 2010 (UTC)
- No, it's PubChem being idiotic (again...) Sulfanylideneiron would be the Fe=S molecule, which is rather different chemical (and probably non-existent) from common-and-garden iron(II) sulfide. Physchim62 (talk) 09:05, 13 March 2010 (UTC)
- What about the vapour phase? I'm aware that in the solid phase, it is an ionic solid, but what is it's vapour phase does it convert to an covalent molecule--Plasmic Physics (talk) 07:16, 6 June 2010 (UTC)
There is some minor naming conflict around that article. Copper monosulfide argument is based on that copper is not simply in 2+ state there, as reflected in the article and its talk; however, this name is not popular on Google books, and copper (II) sulfide is more conventional there. An argument against monosulfide, expressed at my talk, is that prefixes, such as "mono-", should not be used to name ionic compounds. Thoughts? Materialscientist (talk) 04:22, 14 March 2010 (UTC)
- At times like this, we really miss editor User:Axiosaurus who was our chalcogen expert. If one look at the structure of covelite, it is mixed valence at the sulfur part as it contains both isolated S and S2 centers, which most reasonable people (i.e. those who agree with me) would assign as S2- and S22-. Hence the Cu's have two oxidation states. Copper sulfides are described as highly covalent in contrast to what the
idiot,other editor asserts.--Smokefoot (talk) 05:12, 14 March 2010 (UTC) - Maybe copper(I,II) sulfide in parallel with iron(II,III) oxide? Is the IUPAC name simply copper sulfide as the chembox states. Vsmith (talk) 14:00, 14 March 2010 (UTC)
- Yes, in principle copper sulfide refers unambiguously to the compound with formula CuS in stoichiometric nomenclature: obviously, in practice, it is better to provide a bit more information as "copper sulfide" can also refer to the group of CuxSy compounds, of which there are many. Iron(II,III) oxide should really be iron(II) diiron(III) oxide, or iron(2+) diiron(3+) tetra[oxide(2−)] (spinel type) if you want to be really pedantic. Physchim62 (talk) 23:47, 14 March 2010 (UTC)
Copper(I,II) sulfide isn't an ideal name because (according to the article), all the coppers are Cu(I) and the sulfurs are not all sulfides (i.e. S2−).
Copper(I,II) sulfide implies (Cu+)2(Cu2+)(S2−)2, i.e. Cu3S2.
Ben (talk) 15:57, 14 March 2010 (UTC)
- Just going on the structure, it looks like (Cu+)2(Cu2+)(S2−
2)(S2−), or copper(I,II) disulfide sulfide, but I'll bet there's a lot less actual charge separation than that. After all, since when did one see a trigonal planar Cu2+ ion? And shouldn't the sulfide ion be sufficiently reducing to reduce copper(II) to copper(I) in any case? I have no problems in using the stoichiometric name (copper monosulfide) to title the article: if we needed an alternative, we could use copper sulfide (covellite), although I don't think we have any other articles which are disambiguated in that manner. Physchim62 (talk) 17:13, 14 March 2010 (UTC)
- That covellite disamb. works for me (mineralogy background), but not likely supportable from usage by WP:RS. So, how is the compound named in those RS's? I find the IUPAC Name: sulfanylidenecopper [5] a bit much and seldom used (only 46 google hits). Vsmith (talk) 23:10, 14 March 2010 (UTC)
- Sulfanylidenecopper would be wrong, unless we happened to be talking about the Cu=S molecule in the gas phase! We do actually have some support from IUPAC for using the mineral name as a disambiguation: see International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 237. Electronic version., which allows the use of mineral names to define structural types. CAS uses the stoichiometric formula for disambiguation [6], although we would run into problems with displaying titles if we adopted that approach. Physchim62 (talk) 23:47, 14 March 2010 (UTC)
- That covellite disamb. works for me (mineralogy background), but not likely supportable from usage by WP:RS. So, how is the compound named in those RS's? I find the IUPAC Name: sulfanylidenecopper [5] a bit much and seldom used (only 46 google hits). Vsmith (talk) 23:10, 14 March 2010 (UTC)
Web of science hits:
- "Copper monosulfide" 3
- "Copper(II) sulfide" 23
- "Copper sulfide" 871
Looking through the last set, it refers almost equally to either CuS or Cu2S, suggesting that there is no specific name for CuS. Perhaps this is another area where the project can decide the WP convention. Materialscientist (talk) 23:36, 14 March 2010 (UTC)
- Well, I vote for copper monosulfide. Copper sulfide currently is a fleshed-out disambiguation article that discusses the range of compositions for CuSx, including cuprous phases (thanks to Axiosaurus). Alternatively we could rename copper sulfide diambig article as copper sulfides, and then assign copper sulfide to what we currently call copper monosulfide.--Smokefoot (talk) 00:27, 15 March 2010 (UTC)
- Technically, moving articles is not a problem - we can have Copper sulfide and Copper sulfide (disambiguation), as conventional for other topics on WP. Materialscientist (talk) 00:32, 15 March 2010 (UTC)
- On the other hand, we tend not to use names which are ambiguous between two chemical compounds except as the title for an article about a class of compounds (such as copper sulfide is at the moment). Copper monosulfide is unambiguous. The argument that you shouldn't use "mono-" for ionic compounds is completely back-to-front; you shouldn't use Stock notation (with the oxidation number in brackets) unless the compound is substantially ionic (so no manganese(VII) oxide). So "copper(II) sulfide" is wrong on two counts: it's the wrong oxidation state and it implies ionic bonding when the compound is substantially covalent. The downside with "copper monosulfide" as the article title is that we are just about the only people using that name. Physchim62 (talk) 02:13, 15 March 2010 (UTC)
- Technically, moving articles is not a problem - we can have Copper sulfide and Copper sulfide (disambiguation), as conventional for other topics on WP. Materialscientist (talk) 00:32, 15 March 2010 (UTC)
Oxiranes as Subcategory in epoxide[edit]
Just noticed that a separate cathegory exists for Category:Oxiranes which is a subcategory in Category:Epoxides. Any naming nuance I must have missed? If not, I put all oxirane-articles in the epoxide category... L.tak 19:19, 5 April 2010 (UTC)
- The two are synonymous. I agree that it would be best to move articles from Category:Oxiranes to Category:Epoxides. -- Ed (Edgar181) 20:42, 5 April 2010 (UTC)
- done, made a soft direct on Category:Oxiranes... L.tak 21:44, 5 April 2010 (UTC) —Preceding unsigned comment added by L.tak (talk • contribs)
Missing chemistry topics[edit]
I've updated my list of missing chemistry topics - Skysmith (talk) 13:24, 8 April 2010 (UTC)
2-Aminopurine structure[edit]
I discovered a small mistake in the 2-aminopurine structure, the hydrogen on the N7 should be on the N9 and the double bond should be between the C8 and the N7 instead of between the C8 and N9. I am not sure if this is the right place to report this kind of errors but I just registered to wiki and I have no clue how to change this structure myself
Kountraya 18:17, 9 April 2010 (UTC) —Preceding unsigned comment added by Kountraya (talk • contribs)
- It's a matter of convention rather than chemistry where you place the nitrogen, so it's not a serious (i.e., chemically wrong) error. I'm trying to find the recommendations for purine structures to see if we're following them: I'll report back! Physchim62 (talk) 18:28, 9 April 2010 (UTC)
- The "standard" tautomer of purine is 7H, see Panico, R.; Powell, W. H.; Richer, J. C., eds. (1993). A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. p. 168. ISBN 0-632-03488-2. Physchim62 (talk) 18:42, 9 April 2010 (UTC)
Thank you for your quick answer, I guess I have been mislead by the fact that the aminopurine used as a nuctleotide analogue is always bound to the sugar by its N9. Kountraya 19:30, 9 April 2010 (UTC) —Preceding unsigned comment added by Kountraya (talk • contribs)
classification of nitrogen heterocycles[edit]
I think category:nitrogen heterocycles needs to be kind of reformed. It's kind of disorganised. Right now I'm in the process of further breaking it down along the lines of aromatic, aliphatic (it's possible to have a compound which has both), simple or polycyclic, etc. with further information on whether there's a nitrogen that's imine-like, enamine-like, basic sp2, basic sp3, etc. I think this would be a little more informative. John Riemann Soong (talk) 19:22, 21 April 2010 (UTC)
- You might consider upgrading heterocyclic chemistry before the category business. IMHO, your implied talents would have greater impact focusing on this important and needful article. At present, it is a pretty good list, but lacks an overviews on construction approaches, patterns of reactivity, and occurrence. --Smokefoot (talk) 23:39, 21 April 2010 (UTC)
- I would recommend comparing with the categorization of heterocyclic compounds on Commons, which seems to be better organized. -- Ed (Edgar181) 17:18, 28 April 2010 (UTC)
Structure request[edit]
I was wondering if someone might be able to draw a structure for me. The chemical is haematopodin, a pigment found in the mushroom Mycena haematopus (currently at FAC). The structure may be found here, p. 603. Thanks in advance, Sasata (talk) 17:02, 28 April 2010 (UTC)
-
Haematopodin
-
Haematopodin B
Rifleman 82 (talk) 17:15, 28 April 2010 (UTC)
- Wow! That was fast... thanks so much! Sasata (talk) 17:20, 28 April 2010 (UTC)
- Can the pyrrole-nitrogen H be moved closer to the N? Looks like it's floating off a bit. But actually, this is just the easily-dectected compound...the "actual" primary pigment is haematopodin B, which has an NH imine instead of carbonyl at C7 (per quinoline ring-numbering)--see doi:10.1002/ejoc.200700739. It's all an interesting bit of chemical detective-work on those two. Rifleman, could you draw the other also, so we can have comparable structures of both for comparison there? We don't seem to have articles about anything directly related. DMacks (talk) 17:52, 28 April 2010 (UTC)
- Wow! That was fast... thanks so much! Sasata (talk) 17:20, 28 April 2010 (UTC)
About the floating hydrogen - I've looked through the settings and I can't figure out what to tweak. If you are using chemsketch perhaps you can show me? --Rifleman 82 (talk) 01:14, 29 April 2010 (UTC)
- I may have ChemSketch at work...will try tomorrow. I usually use ChemDraw, which puts the H in a less odd position. But when I use (what I think are) the WP:CHEMS recommended settings, the atom labels look out-of-proportion small compared to the bonds and overall structure. See for example File:Ethylenetetracarboxylic_acid_dehydrations.png and File:Ullmann p-diphenoxybenzene.png. Would be great if WP could host actual template files rather than listing a pile of values to enter in a ton of preference panes in each editor program. DMacks (talk) 01:30, 29 April 2010 (UTC)
I found it simpler to rely on ACS settings. Just have to export them at high res? --Rifleman 82 (talk) 01:45, 29 April 2010 (UTC)
I just wanted to remind that we have the page Wikipedia:WikiProject Chemistry/Image Request. Unfortunately, there is however not much traffic… --Leyo 08:06, 29 April 2010 (UTC)
- I used the images and some of the info from Mycena haematopus to start Haematopodin and Haematopodin B. The articles are now stubs, and are in need of some more info on them. --Dirk Beetstra T C 12:20, 29 April 2010 (UTC)
List of commonly available chemicals[edit]
I think that brief ways to produce these chemicals should be listed along with the properties in the list of commonly available chemicals. --Chemicalinterest (talk) 18:50, 5 May 2010 (UTC)
- While well intentioned, the creation of list of commonly available chemicals is ill-advised. The topic is basically original research (who is to decide what is "common"?). What next, uncommon chemicals? --Smokefoot (talk) 22:28, 5 May 2010 (UTC)
Have you seen common chemicals? --Rifleman 82 (talk) 02:19, 6 May 2010 (UTC)
A common chemical is one that you do not have to buy from a chemical supply store or can be manufactured at home, such as chlorine(electrolysis of brine with carbon electrodes[platinum is not needed, carbon is available in Leclanche cells]), sodium sulfate(reaction of lye with battery acid), or calcium oxide (extreme heating of clamshell). --Chemicalinterest (talk) 10:37, 6 May 2010 (UTC)
- Hmm, still close to original research, a lot, if not everything, can be made at home, it may be a lot of work to construct a steroid, but starting from acetic acid, calcium carbide, sodium chloride, water, and maybe even sugar, one can get everywhere. --Dirk Beetstra T C 10:54, 6 May 2010 (UTC)
- Both common chemicals and list of commonly available chemicals are silly and appear to violate the guidelines. "Wikipedia is not a place to publish your own thoughts and analyses or to publish new information." The work is also a form of guidebook, also discouraged: "Wikipedia is not a directory of everything that exists or has existed" WP:NOTDIRECTORY, WP:NOTCATALOG, WP:NOTYELLOW. "The Wikipedia is not a manual, guidebook, textbook, or scientific journal" seeWP:NOTGUIDE etc. Oh well, I might think the effort is ill-advised, but it's just disk space and makes people feel productive.--Smokefoot (talk) 12:23, 6 May 2010 (UTC)
- If the article was so bad, why wasn't the nomination for deletion favored? --Chemicalinterest (talk) 11:43, 18 May 2010 (UTC)
- Both common chemicals and list of commonly available chemicals are silly and appear to violate the guidelines. "Wikipedia is not a place to publish your own thoughts and analyses or to publish new information." The work is also a form of guidebook, also discouraged: "Wikipedia is not a directory of everything that exists or has existed" WP:NOTDIRECTORY, WP:NOTCATALOG, WP:NOTYELLOW. "The Wikipedia is not a manual, guidebook, textbook, or scientific journal" seeWP:NOTGUIDE etc. Oh well, I might think the effort is ill-advised, but it's just disk space and makes people feel productive.--Smokefoot (talk) 12:23, 6 May 2010 (UTC)
Requested move[edit]
At Talk:Aluminium borohydride there is a request to move aluminium borohydride to aluminum borohydride, if anyone wishes to comment. ChemNerd (talk) 17:20, 7 May 2010 (UTC)
- Discussion closed.--Chemicalinterest (talk) 21:52, 19 May 2010 (UTC)
New articles[edit]
- Chloro(dimethyl sulfide)gold(I) --Rifleman 82 (talk) 14:57, 9 May 2010 (UTC)
I'm no chemist, but the long name version of that chemical doesn't seem to follow correct hyphenation conventions. Can anyone take a look? Headbomb {talk / contribs / physics / books} 13:32, 11 May 2010 (UTC)
- most likely 2-(4-Biphenyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole or 2-biphenyl-4-yl-5-(4-t-butylphenyl)-1,3,4-oxydiazole buy it at Sial --Stone (talk) 14:58, 11 May 2010 (UTC)
edit[edit]
{{editprotect}}
Please remove the protection and
Chemicals/Style guidelines Gnevin (talk) 08:18, 21 May 2010 (UTC)
- As far as I can see, it is not protected .. --Dirk Beetstra T C 08:20, 21 May 2010 (UTC)
- Sorry was redirected here . Wikipedia:WikiProject_Chemicals/Style_guidelines is the page Gnevin (talk) 08:24, 21 May 2010 (UTC)
- Note that that page is historical, and no longer in use. The style guidelines are now Wikipedia:Manual_of_Style_(chemistry). Hence, I'm not sure if Wikipedia:WikiProject_Chemicals/Style_guidelines should be unprotected... --Dirk Beetstra T C 10:02, 21 May 2010 (UTC)
- I'm happy for you to revert that action, but I don't believe there is any precedent for fully protecting pages just because they are marked as {{historical}}. — Martin (MSGJ · talk) 10:05, 21 May 2010 (UTC)
Nickel(III) oxide use in secondary cells[edit]
If [[nickel(III) oxide is used so commonly in secondary cells such as ni-cad and nickel metal hydride battery, why is it not well characterized? It doesn't show how it is made industrially either. --Chemicalinterest (talk) 12:49, 28 May 2010 (UTC)
- You don't have to know the composition and structure of a substance to use it. Methylalumoxane is another example of a very widely used substance (for making polythene and related products) whose structure is a bit elusive.
- Do they make it using the way shown in the article? It seemed more like a laboratory process, not an an industrial process. --Chemicalinterest (talk) 14:36, 28 May 2010 (UTC)
- No, I doubt they make it that way! For the electrodes in a secondary cell, the simplest way would be to stick a piece of nickel in a suitable acid solution and then charge it up as the anode of a electrolytic cell at a suitably high potential. That's roughly how you make lead dioxide in a lead–acid battery. Physchim62 (talk) 14:49, 28 May 2010 (UTC)
- Do they make it using the way shown in the article? It seemed more like a laboratory process, not an an industrial process. --Chemicalinterest (talk) 14:36, 28 May 2010 (UTC)
- By the way, is that "No Image" really necessary on Methylalumoxane? --shoy (reactions) 17:11, 28 May 2010 (UTC)
IUPAC name for urea[edit]
Speaking of IUPAC, there's a discussion at Talk:Urea#IUPAC_name that could use knowledgable input. Adrian J. Hunter(talk•contribs) 03:51, 4 June 2010 (UTC)
Expansion of Category:Inorganic compound stubs[edit]
Please take a look at what I did there and give guidelines for whether it is good to expand articles like that. Thank you. --Chemicalinterest (talk) 12:56, 4 June 2010 (UTC)
How do you verify your chembox data ?[edit]
Hi, I just wondering how do you verify your data in the chembox ? No links between data and books or database are provided. Biglama (talk) 13:11, 21 June 2010 (UTC)
Tetraethyllead (known around the world as "tetraethyl lead"[edit]
Appears to be some disagreement about the current article name. The interested are invited to comment at Talk:Tetraethyllead. --Rifleman 82 (talk) 14:43, 26 June 2010 (UTC)
- Note to Plasmic Physics. Re: Your support for a "ramrod" change by Rifleman 82 with no discussion, to the TEL page. You wrote: "Besides, the name change is nearly the same as your proposed name change, albeit with a space removed between lead and tetraethyl. Tetraethyllead is an additively constructed IUPAC name ..." Ever hear of thalidomide? Well, hell, it was "nearly the same", just an enantiomer! (was it the R or S vs.?, I can't remember). "Tetraethyllead" is not the name of the compound developed by Mr. Midgely, nor was it the name DELCO labs, Mr. Kettering, the Ethyl Corp, the DuPont Corp, or General Motors used". Jack B108 (talk) 22:11, 27 June 2010 (UTC)
- While (R)-thalidomide refers to a similiar subtance, (S)-thalidomide, tetraethyllead and tetraethyl lead refers to the exact same substance. You are not comparing appels with appels. What Mr. Midgely named it isn't important in determining the name of the article.--Plasmic Physics (talk) 23:48, 27 June 2010 (UTC)
- See Schlosser's base. Google hits: "LICKOR": 11,500; "Schlosser's base": 146,000. Guess what Manfred Schlosser named it? --Rifleman 82 (talk) 05:11, 28 June 2010 (UTC)
- JackB108's rhetoric suggests that he is over-reacting. We are all in agreement that articles should be highly accessible, and redirects do this smoothly.--Smokefoot (talk) 06:39, 28 June 2010 (UTC)
Ethylene oxide - proposal to pre-announce big edits[edit]
Super-large revision of ethylene oxide, a key chemical compound in terms of its use and production levels. The addition was made by a highly experienced editor. Nonetheless, I suggest that plans such massive (68kb) changes be announced on the relevant talk page with a plan of action. --Smokefoot (talk) 06:39, 28 June 2010 (UTC)
- Information is mostly from the ru.wiki FA article (similar past cases: alkaloid, substituted amphetamine, lactoferrin, diallyl disulfide). Please do check the article. I can't preannounce such things - everything was composed within a day off-line. Materialscientist (talk) 06:52, 28 June 2010 (UTC)
- Well I disagree, respectfully: Not only can you and any of us all pre-announce ideas of super-large edits, such notices would be useful procedurally (minimize wasted editing) while demonstrating good collegiality.--Smokefoot (talk) 15:15, 28 June 2010 (UTC)
- Ah, you were expanding it off-line too? Sorry. I will, but it was really a quick decision to expand it. Materialscientist (talk) 22:52, 28 June 2010 (UTC)
- Well I disagree, respectfully: Not only can you and any of us all pre-announce ideas of super-large edits, such notices would be useful procedurally (minimize wasted editing) while demonstrating good collegiality.--Smokefoot (talk) 15:15, 28 June 2010 (UTC)
Change of ilo.org site structure[edit]
There seems to have been a change in the structure of the ilo.org site. It breaks links with prefix http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/ (and almost surely also shorter prefixes). The new links have /legacy/ instead of /public/. This change invalidates many links (seems like over 200: [7]) pointing to ICSC data sheets of chemicals. I think it would be impractical to change it manually, so maybe some bot could be set up to do the work. --Tomaxer (talk) 23:58, 29 June 2010 (UTC)
- PS Or creating a new template may be a good option... --Tomaxer (talk) 00:01, 30 June 2010 (UTC)
- A lot of them are inserted using templates, so we can do those relatively quickly. It's probably best to make them point to http://www.inchem.org/documents/icsc/icsc/ which has the ICSCs and lots of other useful stuff as well. Physchim62 (talk) 00:04, 30 June 2010 (UTC)
- Hmm, the two templates {{ICSC}} and {{ICSC-ref}} were updated last September, so the ones that are left are the ones which are untemplated... Physchim62 (talk) 00:08, 30 June 2010 (UTC)
- Really, I've forgotten that there already exist these templates. OK, so I'll start to gradually replace those incorrect links by ICSC templates. Thank you. --Tomaxer (talk) 14:24, 30 June 2010 (UTC)
- There seems to be a problem with replacing these templates. I've found out that large part of the articles including these links includes it in the Chembox parameter MSDS with "ICSC nnnn" as the description text. However, none of the two templates can generate such short desc. text and using them would, in my opinion, significantly and unnecessarily expand that field. Also putting it in ref would make it look strange. One solution may be to create one more new template after all with desc. text "ICSC nnnn". Other possibility would be to simply move links in the affected articles from Chembox to the External links section (or entirely remove them, but I don't think this is a good idea). --Tomaxer (talk)
- Really, I've forgotten that there already exist these templates. OK, so I'll start to gradually replace those incorrect links by ICSC templates. Thank you. --Tomaxer (talk) 14:24, 30 June 2010 (UTC)
- Hmm, the two templates {{ICSC}} and {{ICSC-ref}} were updated last September, so the ones that are left are the ones which are untemplated... Physchim62 (talk) 00:08, 30 June 2010 (UTC)
- A lot of them are inserted using templates, so we can do those relatively quickly. It's probably best to make them point to http://www.inchem.org/documents/icsc/icsc/ which has the ICSCs and lots of other useful stuff as well. Physchim62 (talk) 00:04, 30 June 2010 (UTC)
As important as this subject is recently with the Deepwater Horizon oil spill, it could use some attention from someone smarter than I. --shoy (reactions) 17:34, 30 June 2010 (UTC)
Should this user's contributions be seen as unwanted advertisement for “Bioshields”? --Leyo 11:34, 1 July 2010 (UTC)
- Some, but not all. I have left that user's edits at chlorhexidine because they seem to be within our usual practice, but other cases are worse. Nobody has yet contacted the user on the user talk page, that should really be the first step. Physchim62 (talk) 14:39, 1 July 2010 (UTC)
- Stone has already reverted some edits. Is there a talk page template to inform users about “spaming”? --Leyo 21:03, 1 July 2010 (UTC)
- Dunno, Beetstra (talk · contribs) is the person around here who is most involved with conflict-of-interest questions. The account's contributions seem strange to me, but there's very little I can do directly. Physchim62 (talk) 23:02, 1 July 2010 (UTC)
- Stone has already reverted some edits. Is there a talk page template to inform users about “spaming”? --Leyo 21:03, 1 July 2010 (UTC)
Uploaders of chemical structures on Commons are asked to state their opinion here. --Leyo 23:44, 7 July 2010 (UTC)
- BTW: This template is quite similar to Commons:Template:Chemical structure verified. --Leyo 13:20, 12 July 2010 (UTC)
Polyimides[edit]

As above. Uncle G (talk) 04:13, 11 July 2010 (UTC)
- I support the move of Vespel to a proper chemical name, leaving Vespel and Meldin as redirects. I'm not sure about that name though. Poly-(4,4'-biphenyl-(pyromellitic acid)-diimide), proposed at Talk:Vespel is somewhat unwieldy. There should be a better alternative (keywords could be poly, oxydiphenylene, pyromellitimide). Materialscientist (talk) 04:40, 11 July 2010 (UTC)
CheMoBot[edit]
CheMoBot is inserting a {{cascite}} in the chembox when the CAS number doesn't exist in the alledged source, for acridine orange.
- I am going to presume here that someone did check the source in one of the other sources of CAS (the paper version), though indeed it does not exist on commonchemistry.org (CheMoBot is working from our validation indexes, not from the page on commonchemistry.org). That limitation of commonchemistry.org is still a problem, maybe we should find a way to 'separate' that). --Dirk Beetstra T C 07:42, 18 July 2010 (UTC)
A vote on mass addition of video links[edit]
is here. Please participate. Materialscientist (talk) 05:52, 18 July 2010 (UTC)

The diagram at right shows the structure of bongkrek acid. The article gives the IUPAC name as (2E,4Z,6R,8Z,10E,14E,17S,18E,20Z)-20-(carboxymethyl)-6-methoxy-2,5,17-trimethyldocosa-2,4,8,10,14,18,20-heptadioic acid. I'm pretty sure the last part of this is wrong - docosaheptadioic makes no sense to me with a hepta in the centre, and I am thinking it should be docosaheptaenedioic acid (or possibly docosaheptenedioic acid), but I would appreciate a double check from an organic chemist. Thanks, EdChem (talk) 11:25, 30 July 2010 (UTC)
- I'm no organic chemist, but that should certainly be -heptaenedioic. I'll check ChemSpider. Fvasconcellos (t·c) 13:43, 30 July 2010 (UTC)
Thanks... I've been doing some more checking and have run into some more issues with this article. Firstly, the given molecular formula was wrong (given as C28H35O7 when it should have been C28H38O7). Second, the JBC reference refers to bongkrekic acid and not bongkrek acid, giving a (? I think C28H38O7) structure for bongkrekic acid which is clearly different from that shown above (first page of the article clearly shows a bongkrekic acid structure that includes a cyclopentene ring, which the article sources to a PhD thesis). This suggests to me that bongkrek acid and bongkrekic acid are structural isomers. However, the bongkrek acid page lists bongkrekic acid as an alternative name and bongkrekic acid redirects to bongkrek acid, strongly implying they are the same substance. There are sources that refer to the two names as alternatives / synonyms and molecule of the day offers the structure above for bongkrek acid. Help! Is the JBC article just wrong? If it's not, does it support anything in an article on bongkrek acid? Am I just confusing myself? Thanks, EdChem (talk) 15:20, 30 July 2010 (UTC)
- It appears the chemical structure of bongkrekic acid was revised in 1973 (De Bruijn, J.; Frost, D. J.; Nugteren, D. H.; Gaudemer, A.; Lijmbach, G. W. M.; Cox, H. C.; Berends, W. Structure of bongkrekic acid. Tetrahedron (1973), 29(11), 1541-7) The JBC article is from 1970, so it would be expected to have the incorrect structure. The most recent article on the topic that I can find is this one: PMID 10435074. It unambiguously refers to bongkrekic acid and bongkrek acid as synonyms. -- Ed (Edgar181) 15:36, 30 July 2010 (UTC)
Over the last couple of days, I have been writing a new page on the metallocene rhodocene. It's my first attempt at writing a page from scratch, and also my first attempts at uploading any images. I'd appreciate thoughts / comments / suggestions / criticisms, etc. Of course, anyone wanting to jump in and make some changes is welcomed too. EdChem (talk) 13:20, 2 August 2010 (UTC)
- Could you upload the structural formulae in a higher resolution? --Leyo 15:15, 2 August 2010 (UTC)
- Sorry, but no. I don't have a functional version of ChemDraw any more, so I can only make files using existing images I had for other purposes and my skills with images are fairly limited. I suspected that redrawing might be needed, but I figured that a low-res image was better than no image. EdChem (talk) 15:51, 2 August 2010 (UTC)
- I can redo the drawings. The article is relatively specialized, especially considering the needs within WP-chemistry - so many topics that are crying for a general article and so many articles needing re-editing. But rhodocene is nifty and instructive in its own way. --Smokefoot (talk) 17:24, 2 August 2010 (UTC)
- Sorry, but no. I don't have a functional version of ChemDraw any more, so I can only make files using existing images I had for other purposes and my skills with images are fairly limited. I suspected that redrawing might be needed, but I figured that a low-res image was better than no image. EdChem (talk) 15:51, 2 August 2010 (UTC)
Nessler's reagent[edit]
I propose moving this article to potassium tetraiodomercurate(II), with Nessler's reagent redirecting back to a section similarly named. One's a compound, the other's a preparation, and it's misleading to use the chembox thus. --Rifleman 82 (talk) 01:32, 9 August 2010 (UTC)
- I agree, Nessler's reagent should be a section within the potassium tetraiodomercurate(II) article. Also, some of the data in the current article seems questionable to me, such as the melting point being "120-127 °C, 266 K, -77 °F"... unless these are three different temperatures / ranges spanning nearly 200 °C, which would be a melting point without parallel in my experience. EdChem (talk) 03:54, 9 August 2010 (UTC)
That's a bug... when you enter 120-127 °C, it is interpreted as 120-127 = −7 °C. And converted accordingly. --Rifleman 82 (talk) 04:26, 9 August 2010 (UTC)
- OK, thanks, I see how that gets to 266 K, but how does the bug produce −77 °F? EdChem (talk) 04:45, 9 August 2010 (UTC)
Infobox link[edit]
There are very common chemicals that wiki offers a data page for it. I.e. diethyl ether has diethyl ether (data page). Where this happens, the infobox itself should offer such a link (automatically). 18.111.55.86 (talk) 06:27, 10 August 2010 (UTC)
- Click on the header "Supplementary Data Page". --Rifleman 82 (talk) 06:45, 10 August 2010 (UTC)
- but that is not obvious at all. there should be some link closer to the top. 18.111.55.86 (talk) 19:24, 10 August 2010 (UTC)
There is a strange error with the chembox that I cannot track down at the moment. --Leyo 12:37, 17 August 2010 (UTC)
- I guess, the image-related lines were missing that caused the template to take some odd default values. Quick-fixed. Materialscientist (talk) 12:57, 17 August 2010 (UTC)
Redirect for discussion[edit]
The question has been raised as to whether trichloroazane is an alternative name for nitrogen trichloride that actually occurs. Please contribute to the discussion. Uncle G (talk) 07:40, 20 August 2010 (UTC)
Could an administrator delete the Zncl2 redirect page? It is misleading (it has the wrong chemical formula) and the ZnCl2 redirect page is already existing. Thanks! YOSF0113 (talk) 08:44, 24 August 2010 (UTC)
- I think this falls in the category 'common typo's', and redirects are cheap anyway. No need to delete such a redirect (this redirect works for those who type zncl2 as well!). --Dirk Beetstra T C 09:33, 24 August 2010 (UTC)
Example of a bad redirect[edit]
Given the two previous sections, both of which were resolved as "no action needed", I thought it would be an idea to give an example of a truly bad redirect for comparison. From December 2005 until very recently azine redirected to pyridine. Now azine is a possible name for pyridine in Hantzsch–Widman nomenclature but, more importantly, it is also the name of a class of compounds on which we had no article! These are the sort of redirects that need dealing with, not the harmless ones. Physchim62 (talk) 03:09, 26 August 2010 (UTC)
The article 19NorDehydroepiandrosterone appears to be entirely fictional. As far as I can tell, the chemical compound is not mentioned at all in any of the listed references. Is this part of some kind of scam, maybe to boost the sales of bodybuilding supplements that purportedly contain this? ChemNerd (talk) 21:10, 26 August 2010 (UTC)
- Hmm.. how about [8]?--Rifleman 82 (talk) 21:33, 26 August 2010 (UTC)
That's a document written by a company that wants to sell some kind of supplement product containing "19NorDehydroepiandrosterone", not really a reliable source. Of all the references listed in that document, are there any that actually mention this chemical compound? ChemNerd (talk) 22:58, 26 August 2010 (UTC)
- There's one that mentions the diketone in the very title. I don't see why we shouldn't assume good faith for the FDA submission: it's not as if this is an improbable chemical compound. What it does to you is another matter, but I don't intend on trying it to find out ;) Physchim62 (talk) 23:21, 26 August 2010 (UTC)
There are 22 references in the document, and the closest that one can get to having useful verification of the compound is a single reference that only mentions a related compound. I know about the AGF policy, but there is definitely something wrong here. Can anyone verify that this chemical compound has ever been mentioned in the scientific literature or a secondary source independent of commercial interest? ChemNerd (talk) 00:09, 27 August 2010 (UTC)
- I suppose a SciFinder search of the structure would settle the question easily, but I don't have institutional access now.--Rifleman 82 (talk) 04:04, 27 August 2010 (UTC)
- I have done a SciFinder search. A search on the terms "19NorDehydroepiandrosterone", "19-Nordehydroepiandrosterone" and "19NorDHEA" turns up nothing. A search on the chemical structure given in the chembox leads to only two papers that mention this compound:
- Kahan, Ilona L.; Juhasz, Karolin; Mindszenti, Zsuzsa. Lipids of the harderian gland of rabbits. Acta Biologica Academiae Scientiarum Hungaricae (1967), 18(3), 295-301
- Poortman, J.; Vroegindewey-Jie, D.; Thijssen, J. H. H.; Schwarz, F. Relative binding affinity of androstane and C-19-norandrostane-steroids for the estradiol-receptor in human myometrial and mammary cancer tissue. Molecular and Cellular Endocrinology (1977), 8(1), 27-34
- I don't have access to the 1967 Hungarian paper. In the 1977 paper, the compound is just one of many compounds in a list of those tested for activity against estradiol receptors in certain cancer tissues. Overall, I wouldn't say there is anything remotely notable about this compound based on this search. And there is certainly nothing in the scientific literature that backs the content of the article. Consequently, I have nominated the article for deletion: Wikipedia:Articles for deletion/19-Nordehydroepiandrosterone.--Ed (Edgar181) 12:36, 27 August 2010 (UTC)
- I'm not sure why Beetstra removed the verified tags, which placed on the PubChem, and ChemSpiderID parameters. The article does not specify a particular isomer, so I assumed that it was refering to the racemate. The image only represents one isomer, which I stressed in the caption. I verified the racemate identifiers personally, I don't add the PubChem and ChemSpiderID if they don't exist. I'd appreciate a cleared reason than what was given in the edit summary.--Plasmic Physics (talk) 13:28, 27 August 2010 (UTC)
History for Tetrapropylammonium perruthenate is in the disambiguation article TPAP[edit]
The history for Tetrapropylammonium perruthenate is in the disambiguation article TPAP - should it be fixed and what is the procedure? --Christian75 (talk) 11:22, 2 September 2010 (UTC)
Ok, should be fixed. --Rifleman 82 (talk) 11:51, 2 September 2010 (UTC)
Neutronium Categories[edit]
There are two categories associated with neutronium that are of concern: (Category:Neutronium) and (Category:Isotopes of neutronium). Should the former be deleted? Should the Consideration for deletion for the later be reopened? Should the redirect (Isotopes of neutronium) be deleted? Note that User:Headbomb was involved in what appears to be soap boxing by promoting a scientific notion as actual fact. He may still be promoting it. For information on the topic refer to Neutronium.--Plasmic Physics (talk) 12:14, 2 September 2010 (UTC)
- This has been a point of contention at that article for quite a while. Relevant threads are:
- Talk:Neutronium#Disputed: Periodic table (2005)
- Talk:Neutronium#Nnneologism? (2006)
- Talk:Neutronium#Neutronium: The Uncommon Element (2008/2009)
- Talk:Neutronium#References (2009)
- Talk:Neutronium#Infobox (2010)
- My own take is that declaring "neutronium" to be an element was a proposal that was historically noteworthy, but that it isn't considered an element by any significant fraction of the scientific community today. The historical proposal is already covered in its own section in the article. --Christopher Thomas (talk) 17:21, 7 September 2010 (UTC)
- First, I'm not soapboxing, nor promoting a "scientific notion as actual fact", please so please stop misrepresenting me as some POV-pusher. If anything, your actions could very well be construed as forum shopping. There is an ongoing CfD about this, and getting an project unconcerned with the article and related categories like WikiProject Chemicals to chip in the discussion is pretty damn close to canvassing. The relevant projects are WikiProject Elements and to some extent WikiProject Physics and WikiProject Chemistry. Headbomb {talk / contribs / physics / books} 21:45, 7 September 2010 (UTC)
- I've de-indented this by one level, as I am assuming it to be a response to the original poster, not me. With regards to forum-shopping, bringing it to the attention of WP:CHEMS is reasonable, as the debate over whether any significant number of scientists consider "neutronium" to be a chemical element (and so able to have isotopes) is very much within the jurisdiction of this wikiproject (in addition to that of WP:PHYS). This is a common enough state of affairs (look at all of the dark matter and black hole threads that get cross-posted between WT:PHYS, WT:ASTRO, and WT:AST), so calling it forum-shopping is a bit of a stretch. If anyone is acting inappropriately, misrepresenting other users' actions, or editing against consensus, I'm sure it'll rapidly become clear without accusations of forum shopping being needed. --Christopher Thomas (talk) 23:02, 7 September 2010 (UTC)
For anyone interested, the deletion discussion is at Wikipedia:Categories_for_discussion/Log/2010_September_2#Category:Neutronium. -- Ed (Edgar181) 22:44, 7 September 2010 (UTC)
- A nucleus containing only neutrons would not have any electrons bound to it and thus could not be considered as forming an atom, nor could it participate in chemical reactions. So I would not call it a chemical element. Also, according to the article neutronium, only a single isolated neutron would have any significant lifetime.
- A neutron star is gravitationally bound rather than bound by the strong force, so it is not a nucleus as usually understood. Also a neutron star would undoubtedly contain many other particles including protons, so its atomic number would not be zero. JRSpriggs (talk) 23:41, 7 September 2010 (UTC)
- I don't see why its lifetime would matter, we deal with theoretical elements that last microseconds and less already. We also deal with things that are not stable at STP, like post-perovskite, metallic hydrogen, etc. 76.66.197.151 (talk) 05:28, 8 September 2010 (UTC)
- A nucleus containing only neutrons is a nucleus, hence part of nuclear physics, rather than chemistry, which only deals with its consequences in relation to the effect on electrons. As for not taking part in chemical reactions, the formerly named "inert elements" were previously thought to not participate in them either, but were still called elements. (AFAIR, Helium still does participate in chemical reactions, unless ionized). The concept of an element zero / neutronium / neutron-only element recently cropped up in the press-garnering "Chemical Galaxy" popsci periodic table, so this concept does have currency. As all unmade elements are theoretical, that is not a bar to having articles. If there are articles, then categories can be made for them. Even were neutronium/neutron-only element considered obsolete, that would not be a bar to categorization either, since we categorize alot of obsolete science into their own categories. Hell, UFOlogy has their own categories, but a real scientific proposal can't have its own category? 76.66.197.151 (talk) 05:26, 8 September 2010 (UTC)
Related to this subject, I have nominated {{Infobox neutronium}} for deletion at WP:TFD. Icalanise (talk) 15:26, 12 September 2010 (UTC)
Bot for adding images[edit]
A bot could be written for generating models/diagrams for infoboxes based on IUPAC and common name nomenclature. Would anyone support such a request for a bot (until something like jmol is implemented)?Smallman12q (talk) 23:00, 4 September 2010 (UTC)
- Are we lacking images for many infoboxes? I think most of them have had images generated by one of our editors already. Some judgment is necessary, and maybe this should not be automated. --Rifleman 82 (talk) 03:36, 5 September 2010 (UTC)
- I agree with Rifleman 82 here. Often there is a need for a human to draw the structure in an attractive but concise way. Having seen how structure drawing programs can botch a structure when generating them from InChIs etc, I'd rather stick to a human editor for this part. However, a bot to check things like IUPAC naming and InChIs would perhaps be a good idea, using the CAS database as a starting point. Cheers, Walkerma (talk) 04:58, 5 September 2010 (UTC)
- (i) There are 288 articles in the Category:Chemical_pages_needing_a_structure_drawing, mostly inorganic solids (where robots would fail). The category is autoupdated with articles which have chembox and no any picture in it. (ii) I don't think any bot can handle this automatically. Materialscientist (talk) 05:14, 5 September 2010 (UTC)
- I agree with Rifleman 82 here. Often there is a need for a human to draw the structure in an attractive but concise way. Having seen how structure drawing programs can botch a structure when generating them from InChIs etc, I'd rather stick to a human editor for this part. However, a bot to check things like IUPAC naming and InChIs would perhaps be a good idea, using the CAS database as a starting point. Cheers, Walkerma (talk) 04:58, 5 September 2010 (UTC)
Worth saving?[edit]
Does Jensenite have a chance of being salvaged into an actual article (or suitable redirect)? Dabomb87 (talk) 04:22, 13 September 2010 (UTC)
- The entire contents was "Cu3TeO6•2(H2O)" - I'd suggest starting from scratch... Skier Dude (talk 04:24, 13 September 2010 (UTC)
- You can also recreate as a redirect - you all would know the best target. Skier Dude (talk 04:26, 13 September 2010 (UTC)
Nomenclature question...[edit]
I have been asked on my talk page whether "there a difference between rhodocene and 1-rhoda-1,1'-spirobi[pentacyclo[2.2.0.01,3.01,5.02,6]hexane". I have not seen a name like this used for a metallocene before, and am unsure of the answer, so I am asking if anyone here is willing to offer their view. Thanks. EdChem (talk) 08:58, 13 September 2010 (UTC)
- The spiro name implies that there is a single bond from each of the carbon atoms to the rhodium atom. In inorganic nomenclature, it would be bis(cyclopentane-1,2,3,4,5-pentayl-κ5C1,2,3,4,5)rhodium. Rhodocene, on the other hand, is an acceptable shorthand for bis(η5-cyclopentadienyl)rhodium, which is in itself a universally used shorthand for the full systematic name, bis(η5-cyclopenta-2,4-dien-1-yl)rhodium. So the spiro name is incorrect, as it implies ten pairs of bonding electrons; rhodocene, and other names using or implying the η-convention, are correct because they only imply 2×5 carbon atoms taking part in the π-systems that bond to the rhodium (there are six pairs of electrons that take part in metal–ligand bonding in metallocenes). As a final point, the rhodocene names are only appropriate of the low-temperature species; at room temperature, the chemistry is more complex (see article for details). Physchim62 (talk) 12:33, 13 September 2010 (UTC)
- He wrote that article :-P Materialscientist (talk) 12:53, 13 September 2010 (UTC)
- Thanks, Physchim, both for the explanation here and at my talk page. As Materialscientist notes, I wrote the article rhodocene which is probably why the original question was directed to me in the first place. :) It's gratifying to see others have read the page! EdChem (talk) 13:23, 13 September 2010 (UTC)
- He wrote that article :-P Materialscientist (talk) 12:53, 13 September 2010 (UTC)
Someone created this article by copying from the EPA website (so it's not a copyvio), but it's hardly encyclopedic. Someone added a PROD tag to it, but folks here might want to keep an eye on it. Regards, P. D. Cook Talk to me! 19:58, 13 September 2010 (UTC)
Caesium titanate[edit]
Hello chemistry fans.
An article on caesium titanate has recently appeared, written by User:YOSF0113. It gives the formula as Cs2TiO3, but a quick search of crystallographic databases for substances containing only Cs, Ti and O shows no sign of Cs2TiO3. Instead, there are 25 hits, including such stoichiometries as Cs2Ti5O11, Cs0.132TiO2, Cs1.1Ti8O16 and Cs2Ti4O9.
Any suggestions? My guess is that User:YOSF0113 wanted to make a new article, saw existing ones such as barium titanate (BaTiO3), and thought that Ba2+ could easily be replaced by 2Cs+. This is one of the pitfalls of extrapolating without experimental confirmation - WP:OR/WP:SYN?
Ben (talk) 13:12, 19 September 2010 (UTC)
- Well, the stoichiometry Cs2TiO3 makes perfect sense - caesium in its +1 state, titanium in its +4 state, etc. However, the CAS registry number appears to be unassigned, nor do searches for a CAS no. for "caesium titanate" or "cesium titanate" show a result. YOSF0113 is giving specific hazard information, which I suspect is coming from somewhere (as presumably did the CAS number) but with no references it is impossible to check sources. I suggest you ask the editor about sources of information directly. Also, I have serious doubts about the molecule-like structure shown, a true polymeric inorganic salt structure seems much more likely. EdChem (talk) 13:37, 19 September 2010 (UTC)
- Caesium titanate another example of a self-indulgent article, with no serious intent to serve. Such articles are harmless, so long as they are not allowed to imply importance (WP:UNDUE). We used to delete them on grounds of notability. Suggestions: caesium titanate be rewritten much more vaguely or (more efficiently) converted to a redirect.--Smokefoot (talk) 14:08, 19 September 2010 (UTC)
- The stoichiometry Cs2TiO3 doesn't make perfect sense at all. You get BaTiO3 because it forms a nice stable perovskite structure (perovskite itself is CaTiO3), but you can't do that with two M+ cations. Now you can form a material with the stoichiometry Cs2TiO3 – that's just a question of heating equimolar quantities of Cs2CO3 and TiO2 – but is it a single phase? There's a company that sells it, so I'll ask them! Incidentally, because there is a company that sells it, the "compound" has found its way onto many databases: ChemSpider, PubChem, EINECS.
- I would suggest transforming this article into a piece about caesium hexatitanate, Cs2Ti6O13, which is definitely a single phase with a nice layer structure (doi:10.1016/0022-4596(85)90217-8) and appears to have some use in materials science; it is also sold by Aldrich, which is an indication that someone uses it for something. Alternatively, we could write something about caesium titanates in general, but that would need more research. Physchim62 (talk) 15:37, 19 September 2010 (UTC)
- Physchim62, I disagree about Cs2TiO3. I completely agree that that stoichiometry makes no sense for a perovskite, but then it is only Ben's suggestion that YOSF0113 started from barium titanate. I see no reason why Cs2TiO3 is an unreasonable composition for a non-perovskite structure. As you suggest, heating caesium carbonate and titanium dioxide should produce a single phase Cs2TiO3, if it exists, but even if it doesn't that wouldn't be conclusive proof of the non-existence of Cs2TiO3. On what to do with the article, making it about the hexatitanate sounds viable, or keeping it about Cs2TiO3 assuming sufficient reliable information about it can be found. EdChem (talk) 15:56, 19 September 2010 (UTC)
- Sorry, I should have made myself clearer! I wasn't pretending to have proof that Cs2TiO3 doesn't exist – the chemical literature is full of descriptions of compounds that "shouldn't exist" but do! I was merely pointing out that the naïve extrapolation from BaTiO3 is invalid. Personally, I don't think Cs2TiO3 does exist, because these compounds have obviously been studied and we can't find a crystal structure for it, but that's just a personal opinion. The fact that you say that 12158-57-5 appears to be unassigned as a CAS number makes me doubt the existence even more: I can't believe that people just made this CAS number up, when they are quoting it in regulatory situations such as the EINECS list, so that would imply that the number has been deleted from the CAS registry. Physchim62 (talk) 16:23, 19 September 2010 (UTC)
- Physchim62, I disagree about Cs2TiO3. I completely agree that that stoichiometry makes no sense for a perovskite, but then it is only Ben's suggestion that YOSF0113 started from barium titanate. I see no reason why Cs2TiO3 is an unreasonable composition for a non-perovskite structure. As you suggest, heating caesium carbonate and titanium dioxide should produce a single phase Cs2TiO3, if it exists, but even if it doesn't that wouldn't be conclusive proof of the non-existence of Cs2TiO3. On what to do with the article, making it about the hexatitanate sounds viable, or keeping it about Cs2TiO3 assuming sufficient reliable information about it can be found. EdChem (talk) 15:56, 19 September 2010 (UTC)
- Caesium titanate another example of a self-indulgent article, with no serious intent to serve. Such articles are harmless, so long as they are not allowed to imply importance (WP:UNDUE). We used to delete them on grounds of notability. Suggestions: caesium titanate be rewritten much more vaguely or (more efficiently) converted to a redirect.--Smokefoot (talk) 14:08, 19 September 2010 (UTC)
Hello, I'd like to bring the above article to GA status, but have never done so with an article on a chemical (I admit, I'm only interested because it's made by fungi). I'd highly appreciate it if any members of this project could have a quick look and see if there's anything obvious I'm missing that should be included. Thanks! Sasata (talk) 16:09, 19 September 2010 (UTC)
- From a quick look, issues that occur to me / things to consider:
- The compound has (E) and (Z) isomers, but which comments apply to which isomers is not specified
- I suspect that the laboratory synthesis of the compound(s) are described in the literature; these should be described
- Is there undue weight on the information about the fungus, etc, as opposed to the compound?
- Is it worth considering a section on the "Properties" of the compound, or maybe a "History" section?
- The references are not consistently formatted
- Does the lede summarise all of the article?
- EdChem (talk) 16:30, 19 September 2010 (UTC)
Chemicals articles have been selected for the Wikipedia 0.8 release[edit]
Version 0.8 is a collection of Wikipedia articles selected by the Wikipedia 1.0 team for offline release on USB key, DVD and mobile phone. Articles were selected based on their assessed importance and quality, then article versions (revisionIDs) were chosen for trustworthiness (freedom from vandalism) using an adaptation of the WikiTrust algorithm.
We would like to ask you to review the Chemicals articles and revisionIDs we have chosen. Selected articles are marked with a diamond symbol (♦) to the right of each article, and this symbol links to the selected version of each article. If you believe we have included or excluded articles inappropriately, please contact us at Wikipedia talk:Version 0.8 with the details. You may wish to look at your WikiProject's articles with cleanup tags and try to improve any that need work; if you do, please give us the new revisionID at Wikipedia talk:Version 0.8. We would like to complete this consultation period by midnight UTC on Monday, October 11th.
We have greatly streamlined the process since the Version 0.7 release, so we aim to have the collection ready for distribution by the end of October, 2010. As a result, we are planning to distribute the collection much more widely, while continuing to work with groups such as One Laptop per Child and Wikipedia for Schools to extend the reach of Wikipedia worldwide. Please help us, with your WikiProject's feedback!
For the Wikipedia 1.0 editorial team, SelectionBot 22:14, 19 September 2010 (UTC)
WPChem worklist articles have been selected for the Wikipedia 0.8 release[edit]
Version 0.8 is a collection of Wikipedia articles selected by the Wikipedia 1.0 team for offline release on USB key, DVD and mobile phone. Articles were selected based on their assessed importance and quality, then article versions (revisionIDs) were chosen for trustworthiness (freedom from vandalism) using an adaptation of the WikiTrust algorithm.
We would like to ask you to review the WPChem worklist articles and revisionIDs we have chosen. Selected articles are marked with a diamond symbol (♦) to the right of each article, and this symbol links to the selected version of each article. If you believe we have included or excluded articles inappropriately, please contact us at Wikipedia talk:Version 0.8 with the details. You may wish to look at your WikiProject's articles with cleanup tags and try to improve any that need work; if you do, please give us the new revisionID at Wikipedia talk:Version 0.8. We would like to complete this consultation period by midnight UTC on Monday, October 11th.
We have greatly streamlined the process since the Version 0.7 release, so we aim to have the collection ready for distribution by the end of October, 2010. As a result, we are planning to distribute the collection much more widely, while continuing to work with groups such as One Laptop per Child and Wikipedia for Schools to extend the reach of Wikipedia worldwide. Please help us, with your WikiProject's feedback!
For the Wikipedia 1.0 editorial team, SelectionBot 23:49, 19 September 2010 (UTC)
The section Psoralen#Biosynthesis refers to two diagrams that are missing. Looking at the history, it looks like the diagrams have been deleted. Would an admin please have a look at them and see whether they are worth re-creating? Alternatively, the section needs a re-write, preferably by someone with good structural diagrams relating to shikimate biosynthesis from which to work. Thanks. EdChem (talk) 07:14, 20 September 2010 (UTC)
- Although I didn't do this section of the article, and I'm not an admin, I'd be happy to try creating some diagrams if the old ones can't be rescued or are wrong. Walkerma (talk) 13:06, 20 September 2010 (UTC)
- I have temporarily restored the two files, File:Psoralen Biosynthesis 1.jpg and File:Psoralen Biosynthesis 2.jpg, if anyone wants to take a look. -- Ed (Edgar181) 13:27, 20 September 2010 (UTC) Update: Because the files do not have the required permissions I have had to redelete them. New images can be generated from the information in Rifleman's link below. -- Ed (Edgar181) 11:45, 2 October 2010 (UTC)
- It seems (from threads at their uploader's talk page) that they were deleted due to lack of licensing information, so creating new diagrams from scratch conveying the same information as the old ones is probably best. The old ones cite a book reference, so there will always be a concern that they're cleaned-up scans (though I consider that unlikely myself). --Christopher Thomas (talk) 18:01, 20 September 2010 (UTC)
Just as well. They can be improved to meet prevailing community standards with regard to file format, resolution, etc. in the process.--Rifleman 82 (talk) 19:18, 20 September 2010 (UTC)
- These are definitely not book scans, we've seen plenty of those on this project! But they can be redrawn without too much trouble and with a clear license, so let's do that. Physchim62 (talk) 20:14, 20 September 2010 (UTC)
- Does anyone have access to the book? That would be best, rather than redrawing what has been redrawn from a book. --Rifleman 82 (talk) 20:46, 20 September 2010 (UTC)
- The problem is that if the original contributor doesn't step forward and say "I drew these, and release them under (some acceptable license)", the images might still be declared unacceptable. I agree that blessing the existing images is the path of least work, but redrawing them is IMO likely to be the path of least red tape. --Christopher Thomas (talk) 21:43, 20 September 2010 (UTC)
- I actually meant that they should still be redrawn, but with reference to the actual book to reduce the chance for errors. It's available on Google Books [9], so if anyone can help... I could but I prefer that someone who actually understands do it. --Rifleman 82 (talk) 22:48, 20 September 2010 (UTC)
- The problem is that if the original contributor doesn't step forward and say "I drew these, and release them under (some acceptable license)", the images might still be declared unacceptable. I agree that blessing the existing images is the path of least work, but redrawing them is IMO likely to be the path of least red tape. --Christopher Thomas (talk) 21:43, 20 September 2010 (UTC)
GA and A-class[edit]
Would someone please explain how GA-status and A-class interact? Rhodocene has just been promoted to WP:GA status, but is still listed as B-class. I've been trying to figure out what is separate / different about A-class that isn't covered by GA, but I'm struggling. Thanks. EdChem (talk) 04:00, 23 September 2010 (UTC)
Sunday puzzle - CO2 scrubber material for astronauts[edit]
I was going to mention in lithium hydroxide that it is/was used on the Apollo missions and related space ventures. But does anyone here know? "CO2 scrubber" redirects to Rebreather, and makes no mention of use in space.--Smokefoot (talk) 19:56, 26 September 2010 (UTC)
- I think it was lithium peroxide that was used: I'll see if I can find a reference or that. Physchim62 (talk) 20:55, 26 September 2010 (UTC)
- Yep, it's the peroxide, according to Greenwood & Earnshaw: the peroxide actually gives you oxygen back as well as absrobing the CO2. Physchim62 (talk) 20:58, 26 September 2010 (UTC)
Covalent network solids[edit]

Can anyone explain how covalent network structures terminate, how is the nature of bonding different in the surface as opposed to the bulk structure. If you can, please use diamond and quartz as examples.--Plasmic Physics (talk) 10:45, 26 September 2010 (UTC)
- Diamond probably has C–H bonds on its surface. Quartz has surface silanol groups, Si–OH, but these are surface ionisable groups and so there is a mix of Si–O−, Si–OH, and Si–OH2+ groups on the surface. The distribution depends on the environment. The point of zero charge occurs when the surface concentrations of Si–O− and Si–OH2+ are equal. EdChem (talk) 11:02, 26 September 2010 (UTC)
- Can look up my files, but what I recall is that first, these surfaces reconstruct, i.e. in ideal structure (which is experimentally observed in high-quality clean experiments), the bonding in the last layer differs from that in the bulk, with various dimer configurations present or not depending on the surface orientation (the jargon for that is axb reconstruction, where a and b are integers, e.g. 2x1). Their chemical termination is a function of the surface preparation. In diamond and silicon, typical terminations are C-H, C=O, CH2 and C-O-C. The reconstruction may create supercells on the surface with the "lattice constant" much larger than the bulk lattice constant. Materialscientist (talk) 11:16, 26 September 2010 (UTC)
- now that is a great question seldom asked - in classes and books, chemists mainly discuss the interior of crystals, not their exteriors. For molecular solids reconstruction involves no new bonds, but for nonmolecular solids, including boring old NaCl, things are very different.--Smokefoot (talk) 16:32, 26 September 2010 (UTC)
- Can look up my files, but what I recall is that first, these surfaces reconstruct, i.e. in ideal structure (which is experimentally observed in high-quality clean experiments), the bonding in the last layer differs from that in the bulk, with various dimer configurations present or not depending on the surface orientation (the jargon for that is axb reconstruction, where a and b are integers, e.g. 2x1). Their chemical termination is a function of the surface preparation. In diamond and silicon, typical terminations are C-H, C=O, CH2 and C-O-C. The reconstruction may create supercells on the surface with the "lattice constant" much larger than the bulk lattice constant. Materialscientist (talk) 11:16, 26 September 2010 (UTC)
- The topic certainly comes up often in semiconductor research. Surface effects can give you defects that trap carriers, and bonds that don't match the chemistry of the bulk material add states inside the bandgap that can cause problems. The latter caused considerable difficulty with silicon nanocrystal research until they figured out what was happening.
- User:Materialscientist gives a good description of what happens when you have a crystal edge that's been passivated by its environment. In vacuum, you can get dangling bonds that stay around for a while (due to absence of relaxation mechanisms). Among other things, this is what causes vacuum cementing (touching two cleaved surfaces back together causes them to stick as some of the dangling bonds react with each other). --Christopher Thomas (talk) 21:02, 26 September 2010 (UTC)
Interisting replies. So, if the solid is created under a vacuum, it is essentially a highly reactive polyradical that reacts with just about any surface it contacts; being under atmospheric conditions passivates the radical moeities? Are there some covalent network solids that are not polyradicals when created in a vacuum, and can exist in a stable unpassivated form under atmosphere.--Plasmic Physics (talk) 21:26, 26 September 2010 (UTC)
- Yes, dangling bonds remain and the solid surface is highly reactive in vacuum. On the fly, I don't know any inorganic covalent solid which would heal itself. Diamond could, at elevated temperature, by transforming into graphite, but the required reconstruction would be too complex and involve many atomic layers - it would not be a usual reconstruction but rather a heterostructure of diamond and some graphite-like structure. Materialscientist (talk) 22:19, 26 September 2010 (UTC)
- Possibly. Non-covalent substances like metals almost certainly can do this. Some covalent substances may self-passivate to some degree by forming bonds of different types than those normally found within the crystal. I'd expect diamond and silicon to do this to some degree; at minimum diamond should be able to form graphite-style pi bonds at the surface, though it'll still be reactive (just without dangling bonds). That said, I defer to User:Materialscientist's expertise (it's possible I'm mistaken). --Christopher Thomas (talk) 21:49, 26 September 2010 (UTC)
I'm doing a Bachelor of nanoscience degree, so I find this really interisting. On the quartz note: is the surface structure responsible for the hydrophilicity of laboratory glassware? Is it related to the catalytic behaviour of zeolites?--Plasmic Physics (talk) 23:24, 26 September 2010 (UTC)
- ABSOLUTELY (and then more so!) sorry for shouting ;) If you want to make your laboratory glassware much less hydrophilic, just rinse it around with a bit of trimethylsilyl chloride: if you're brave, you can even add a drop or two of (bone dry) triethylamine to the mix. Physchim62 (talk) 23:45, 26 September 2010 (UTC)
- Well... that your glass is hydrophilic is a traditional check that it is clean (water sheets off easily). That you don't have lipophilic organic crap left on the surface. I have seen an alternate procedure to cap the hydroxyls with TMS-Cl by putting everything in an oven, with a few mL of TMS-Cl in a beaker. --Rifleman 82 (talk) 16:21, 2 October 2010 (UTC)
- A well-known chemical company will even supply you with a ready-made solution for coating glassware with TMS groups: apparently the protein crystallographers use it quite a bit – or so a protein crystallographer told me when he suggested this as a solution to my adsorption problem (an inorganic anion that was adsorbing onto the glass surface to such an extent that it was messing up my analyses). Physchim62 (talk) 16:44, 2 October 2010 (UTC)
- Well... that your glass is hydrophilic is a traditional check that it is clean (water sheets off easily). That you don't have lipophilic organic crap left on the surface. I have seen an alternate procedure to cap the hydroxyls with TMS-Cl by putting everything in an oven, with a few mL of TMS-Cl in a beaker. --Rifleman 82 (talk) 16:21, 2 October 2010 (UTC)
Cojugation of pi bonds can only take you so far, in terms of dispersing the radical electrons, like a game of musical chairs. A graphene sheet may not have a polyradical surface, but it has an edge, giving you the same problem in a different light.--Plasmic Physics (talk) 23:35, 26 September 2010 (UTC)
- The "edge problem" is a big issue when you come you use practical graphite powders, rather than just thinking in terms of the idealized structure. To add a comment to you question "are there any solids…", I don't think so, but I think the nearest you'd come is with one of the allotropes of boron – these are already "electron-deficient" for normal covalent bonding, so I would imagine that they would accomodate the surface better. In fact, the accomodate surfaces so well that it is extremely difficult to crystallize them! That's the trade-off, I'm afraid… Physchim62 (talk) 23:45, 26 September 2010 (UTC)
- Think real. It is extremely difficult to bond graphene to a crystal, even in theory. "Graphite-like" on diamond always means short-range order, i.e. local sp2 reconstructions, it is quite messy in the long range. Any amorphous surface can self-heal. Crystalline boron has several allotropes, some with rather complex structure. I know some of them do have ordered surface, but I know no evidence that they can self-heal without hydrogen. Materialscientist (talk) 00:24, 27 September 2010 (UTC)
Ants[edit]
I inadvertantly boiled ants, when they decided that a the cavity within a kettle lid is a convenient nesting site. I only noticed it after the kettle has boiled. Now the kettle is contaminated with a persistent and repulsive smell and taste. I've rinsed it with fresh water repeatedly, I've boiled a detergent solution in it, ultimately I can't seem to remove this contaminant. Any ideas what the chemical is and how to remove it, I thought it could be formic acid, but considering how difficult it is to remove, this seems unlikely. In my opinion, it smells like a mixture of acrylate, bread mould and dirt. Any water boiled in it gains that smell and taste which, has not happened before this event.--Plasmic Physics (talk) 11:41, 17 October 2010 (UTC)
- My best suggestion is to soak the whole thing in bleach. Also, the reference desk (WP:RD) is probably the best location for questions like this. --Christopher Thomas (talk) 17:56, 17 October 2010 (UTC)
Shoe Goo[edit]
The article Shoe Goo could stand to be looked at by somebody with a chemistry background. The material is called variously a polymer, a polyurethane-based product, and individual chemical components are mentioned. Without getting overly complex, would it be possible to make sure that the product is being characterized properly? Thanks. Carrite (talk) 20:17, 17 October 2010 (UTC)
Sounds like a polyurethane which crosslinks slowly when applied. Toluene and naphtha are merely solvents to dissolve everything. --Rifleman 82 (talk) 20:46, 17 October 2010 (UTC)
Definition of applications[edit]
I was reading the article Iridium and noticed that it has a section discussing C-H activation with a reaction scheme. This particular reaction is not and has not been used for anything, aside from generating some nice publications. So I was going to move this bit to the compounds section, since it is not an application. The section in question is "Carbon–hydrogen bond activation (C–H activation) is an area of research .."
Now the more general question/assertion: we dont define "research" as applications, do we? Agreeing or discussing the definition(s) of application has broader implications for Wikipedia-Chemistry. My vote is that under applications, when we discuss compounds/elements that are actually being used, usually commercially but not always. But I get the feeling that others might disagree.--Smokefoot (talk) 00:25, 23 October 2010 (UTC)
- IMO, "laboratory research" applications should not be mixed with industrial ones, but many cases are to be treated individually. Imagine a compound (say DPPH, though it might be a bad example) which is not studied on its own, and is not used by ordinary people, and is sold as a chemical rather than tool, but is widely used as a "tool" in the lab for some specific purpose (e.g. DPPH is the most common EPR standard + radical marker). Materialscientist (talk) 00:38, 23 October 2010 (UTC)
- C-H activation is a very specialised topic and should not be mentioned on the iridium page. Ideally there should be a separate page on iridium compounds and then iridium C-H activation should be handled by the organoiridium page. With respect to applications: if a compound is commercially available it should be mentioned. With respect to commercially relevant reactions: aside from bulk chemistry it is virtually impossible to obtain information (trade secrets, if a patent exists it does not mean it is used in production, is the Negishi coupling used in industry? ). Academic research can be handled under scope. Finally: I would hate to see science banned from Wikipedia, there is more in life than just useful applications V8rik (talk) 23:24, 23 October 2010 (UTC)
- I think a lot depends on the context. I agree that applications such as the C-H activation section are too specialised for the iridium article, but if its an article like 1,1'-Bis(diphenylphosphino)ferrocene, then the applications are likely to be laboratory-based. I've always taken the view that "if plenty of other people (besides the originators) use it for that, then it counts as an application". For example, plenty of people besides Evans use oxazolidinones as chiral auxiliaries, so it's appropriate to mention that application on the 2-Oxazolidone page. But if it's mainly just used by the group that developed it, it's not really an application - it's just scientific research.
- A couple of other comments: (1) I don't think C-H activation is very specialised any more. At our NERM conference in June, about half of the speakers in the organic chemistry sessions were talking about their work in that area, and a glance at Organic Letters will show how mainstream it's become. My impression is that it's currently a very hot area in organic, at least in the US. (2) The borylation (is that the right word, v8rik?) of arenes with an iridium catalyst, as described here, is beginning to be used - it's probably not worth mentioning on the iridium page, but it may be a significant application of this type of chemistry. Walkerma (talk) 02:41, 24 October 2010 (UTC)
Phase Diagrams[edit]
I think that it is a good idea to accumulate phase diagrams for the elements and maybe simple substances, as wikipedia has surprisingly few.-Plasmic Physics (talk) 07:27, 26 October 2010 (UTC)
Ates[edit]
Many of us are careful to not allow nomenclature detract from the functionality of articles. But the issue comes up all the time. For example, Wikipedia has a fine article on phosphate. In the lead paragraph, it would probably be unhelpful/distracting to remind readers that the term "phosphate" is not reserved for oxides, e.g. PCl6-. We can be sure that >99.99% of phosphate readers are disinterested in the halophosphates, I assume. I ran into this problem in searching for titanates, and encountering [TiCl6]2-. So the article titanate begins with a short disclaimer that the term can be used more broadly but the article is about the oxides. IUPAC does not apparently define phosphate, but what about "-ates"? — Preceding unsigned comment added by Smokefoot (talk • contribs) 15:12, 30 October 2010 (UTC)
- IUPAC defines phosphate as PO3−
4 on page 325 of International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. Electronic version.. The use of "-ate" is on page 72 of the same reference: in formal terms, it the suffix is used in additive nomenclature to denote any heteropolyatomic anion ("-ide" is used for monoatomic and homopolyatomic anions). Physchim62 (talk) 18:23, 30 October 2010 (UTC)
IMHO the name hexachlorophenol is incorrect, because it is not a phenol anymore. The CAS database lists the compound as 2,3,4,4,5,6-hexachloro-2,5-cyclohexadien-1-one. Hexachloro-2,5-cyclohexadienone would be a shorter alternative. Any comments? --Leyo 14:48, 5 November 2010 (UTC)
- The name doesn't bother me to much – we can always add a sentence saying that it's not really a phenol – but the compound doesn't seem to be at all notable, evn by our low standards of notability! Physchim62 (talk) 15:07, 5 November 2010 (UTC)
- The name bothers me - a phenol is an aromatic alcohol. This compound is neither aromatic nor an alcohol, it's a cyclic ketone. Unfortunately, if there are multiple RS that use the name, the fact that it is chemically inappropriate would not be a reason not to at least record it as an alias. Also, I share Physchim62's concerns on notability, there doesn't seem anything particularly significant about the compound asserted in the article. EdChem (talk) 15:19, 5 November 2010 (UTC)
- "Hexachlorophenol" is a valid synonym in the sense that this name is used in the scientific literature (one of the earliest references is a US patent titled "Hexachlorophenol"). But I agree that it is certainly misleading. As for the notablity, SciFinder returns 86 hits on the compound which is more than for many other compounds that we have articles for. I haven't looked closely to see if many of these 86 hits are more than passing references though. -- Ed (Edgar181) 15:28, 5 November 2010 (UTC)
I notice that most of the articles listed at "What links here" for alcohol really should link to alcoholic beverage. Is there any good and easy way of fixing these using one of Wikipedia's many tools or gadgets? ChemNerd (talk) 16:12, 5 November 2010 (UTC)
- Wow, you're right. I doubt people browsing the Drew Barrymore page are really looking for an education in organic chemistry. One way to fix this might be to load a list of pages that link to alcohol in AWB, filter out all pages with chemistry categories, then update the link (checking each change for appropriateness, of course.) I'll take a stab at doing that on Thursday if no one else volunteers or can think of a better approach. 28bytes (talk) 03:12, 10 November 2010 (UTC)
- I was thinking of doing an AWB run as well. I guess it's a big problem (I haven't actually looked, it scares me too much!) so help from anyone who runs AWB would be appreciated! If we all do a run until we get too comatose, we should be able to fix it quite quickly... Physchim62 (talk) 03:31, 10 November 2010 (UTC)
Okay... I'll try to dust off my bot account and do a run. --Rifleman 82 (talk) 03:58, 10 November 2010 (UTC)
OKay, this is not a trivial problem. There are 3k articles, and it's taking some time to filter out the chemistry articles. I'll work on it over the week. Advantage is that with a bot flag, we don't clutter up watchlists. --Rifleman 82 (talk) 04:14, 10 November 2010 (UTC)
- Thanks 28bytes, Physchim and Rifleman. If there is anything I can do to help (without access to AWB), let me know. ChemNerd (talk) 13:53, 10 November 2010 (UTC)
True pyrrole?[edit]
Check out this pair: True Pyrrole, not to be confused with pyrrole. --Smokefoot (talk) 00:49, 10 November 2010 (UTC)
- The article's creator is a good faith contributor, so I don't see how this is the result of anything but some misconception. I think it should just be redirected to pyrrole. -- Ed (Edgar181) 12:22, 10 November 2010 (UTC)
Is "true pyrrole" supposed to be a radical?
Ben (talk) 16:37, 10 November 2010 (UTC)
- It has to be one IMHO. Strangely, PubChem has an entry for this compound. --Leyo 17:25, 10 November 2010 (UTC)
Ben (talk) 19:12, 10 November 2010 (UTC)
- Whilst the pyrrolyl radical might exist under certain conditions, as far as I can see "True pyrrole" is just made up. I've asked him for references, but would be suprised if any were forthcoming. Couldn't find anything on WoS or Google Scholar. Chris (talk) 21:40, 10 November 2010 (UTC)
user:Plasmic Physics[edit]
keeps adding rare IUPAC names (some are never used and have almost zero Google hits) to chemistry articles. I would be glad to know the stance of the project on that. Materialscientist (talk) 01:50, 4 June 2010 (UTC)
I've tried to tell him a few times, but there is no reply. I think we should just revert it on sight. --Rifleman 82 (talk) 10:38, 4 June 2010 (UTC)
- I left a note on Plasma Physics's talk page, asking him/her to discuss things with us.
- Is failure to engage in discussion grounds for blocking?
- Ben (talk) 11:19, 4 June 2010 (UTC)
- Not really. His editing has to be disruptive to merit a block and, from what I've seen, the user is quite there yet. But by all means revert, under the BRD principle. Physchim62 (talk) 11:31, 4 June 2010 (UTC)
OK, I've asked him to come and discuss this here. For my part, whilst these edits are generally factually correct they do not add anything to the article (the IUPAC names can go in the infobox, by all means), and are distracting.... The thing is, nobody actually uses these names, and as far as I'm concerned we shouldn't go filling up the lead paragraph with lots of irrelevant information. Chris (talk) 13:31, 4 June 2010 (UTC)
- If they have a place, they could go into the 'othernames' field in the Chembox. That said, they are often too long, mostly unused etc., and for many compounds more IUPAC names are possible. The only one that would be of interest is the Preferred IUPAC name (new parameter, PIN field in the main body of the chembox), but I don't think there are definite decisions on that, yet. Although I do see the effort, and in a way the info is useful (if we want to be a source of chemical information (and we are, eyes are also upon us ..), then by all means, it should be possible to find chemicals by all possible means, and that includes as many 'identifiers' as possible, even if 'no-one' uses them.[citation needed] (but then still, it does not mean they need a prominent place in the page). --Dirk Beetstra T C 13:41, 4 June 2010 (UTC)
Here's a transclusion of the three-way multiple-talk-page discussion between Plasma Physics, Chris, and me:
| The original thread on Plasma Physics's talk page:
Dear Plasma Physics, Have you seen the latest discussion about your edits at Wikipedia talk:WikiProject Chemicals#user:Plasmic Physics? There are many editors who find you addition of (supposedly) IUPAC names unhelpful, but none (except you) who support them. It would benefit Wikipedia, and in particular its chemistry articles, if you would at least engage in discussion on the matter. Ben (talk) 11:16, 4 June 2010 (UTC)
|
- I have been trying to join the discussion here, but was unable due to edit conflicts. Mean while argument is already apparent. I must make a note, the IUPAC names are not explicitly stated in the IUPAC PRNOC (2004) as it is not a comprehensive catelogue, but simply a set of rules by which the names can be constructed. The names themselves are assembled by a third party following these rules. If said third party is not absolutely certain of the correctness of a name, then it does not qualify for addition to wikipedia. Google does not provide every result on a chemical search, sites of chemical suppliers uses the names mostly when Google doesn't. So Google should not be trusted so completely to provide all citations, it is not absolutely reliable for searching obscure IUPAC names.--Plasmic Physics (talk) 21:34, 4 June 2010 (UTC)
- Then please understand that you do appear as this third party, diligently trying to construct names, which nobody knows and nobody uses and which are therefore opposed because this contradicts the basic rule of wikipedia - document notable and reliable information. I believe we all here honestly aim to improve wikipedia, including yourself. Please, think constructively. Some conventions of this project are hard to understand, but are easy to live with, and the current consensus is clearly against those names. Materialscientist (talk) 23:33, 4 June 2010 (UTC)
- I have been trying to join the discussion here, but was unable due to edit conflicts. Mean while argument is already apparent. I must make a note, the IUPAC names are not explicitly stated in the IUPAC PRNOC (2004) as it is not a comprehensive catelogue, but simply a set of rules by which the names can be constructed. The names themselves are assembled by a third party following these rules. If said third party is not absolutely certain of the correctness of a name, then it does not qualify for addition to wikipedia. Google does not provide every result on a chemical search, sites of chemical suppliers uses the names mostly when Google doesn't. So Google should not be trusted so completely to provide all citations, it is not absolutely reliable for searching obscure IUPAC names.--Plasmic Physics (talk) 21:34, 4 June 2010 (UTC)
The other point to remember is that PRNOC(2004) is a proposal: it has not been adopted, and will not be adopted in exactly those words. The public draft is internally inconsistent: if you look carefully, it gives two different names for acetone (the second being propan-2-one). We don't know when the new rules are coming out – 2011 is a date I've heard from a very well placed source, but that is not an official announcement – until then, PRNOC2004 is just an idea as to the direction that things are going rather than a set of rules to be applied blindly. Physchim62 (talk) 01:34, 5 June 2010 (UTC)
- What about IUPAC names that are citable? That is what I'm most concerned with - if I can cite a IUPAC name, does it still not warrant a place in the article?--Plasmic Physics (talk) 02:52, 5 June 2010 (UTC)
- Put it in the box, not in the text. Though you might want to save your effort til the Preferred IUPAC names recommendations are approved. --Rifleman 82 (talk) 03:01, 5 June 2010 (UTC)
- Alright, so I will put it under the other names section and cite it. Although, I don't see any reason wait - there is no sense in allowing one IUPAC name, if the article has one, while not allowing another, especially if it is disallowing indefinitely. An addition of a cited alternative name does not harm wikipedia's integrity does it? When who knows when the PIN recommendations are approved, a switch can be carried out by choosing which of the IUPAC names to place in the IUPAC section for the template. By no means should the loser be removed, as it was still and maybe is still used in some sources.--Plasmic Physics (talk) 08:35, 5 June 2010 (UTC)
- I have a concern about citing: often, it is possible to find one-two obscure journal articles which use a certain rare name. This won't do. Materialscientist (talk) 08:40, 5 June 2010 (UTC)
- Alright, so I will put it under the other names section and cite it. Although, I don't see any reason wait - there is no sense in allowing one IUPAC name, if the article has one, while not allowing another, especially if it is disallowing indefinitely. An addition of a cited alternative name does not harm wikipedia's integrity does it? When who knows when the PIN recommendations are approved, a switch can be carried out by choosing which of the IUPAC names to place in the IUPAC section for the template. By no means should the loser be removed, as it was still and maybe is still used in some sources.--Plasmic Physics (talk) 08:35, 5 June 2010 (UTC)
- Apart from Materialscientist's and anyone else's concerns, Plasmic Physics: Can you please then fix this then? Specifically, move your text to the box. --Rifleman 82 (talk) 09:03, 5 June 2010 (UTC)
- Working on it.--Plasmic Physics (talk) 11:45, 5 June 2010 (UTC)
- Have a look at my latest edit to nitric acid, is this the general idea?--Plasmic Physics (talk) 13:03, 5 June 2010 (UTC)
The source you've provided for the name "oxazinic acid" for nitric acid doesn't look very reliable: "oxoazinic acid potassium" at molport.com. There's some very questionable data there, notably the structure and formula, which seem to imply a 1:1 mixture of potassium hydride and nitric acid. Not a mixture that would hang around for very long:
- KH + HNO3 → KNO3 + H2
Have you read WP:RS?
Ben (talk) 13:31, 5 June 2010 (UTC)
- This editor has demonstrated a consistent disinterest in helpful editing at least with respect to names and chemistry. Instead, PlasmicPhysics is engaged in some sort of asinine, litigious game. I recommend that the community continue to revert this editor's work upon sight, as many here have done over the past few years. I am unconvinced that every possible ultra-obscure name even merit inclusions in the ChemBox.--Smokefoot (talk) 14:39, 5 June 2010 (UTC)
- To everyone who is actually interested in finding a solution rather than critising my motives: If it's a problem with my choice of citation, then what about [[10]]? It can be found under Chemical IUPAC Name. It is not uncommon for compounds to be discribed as a product of their reactants. The previous citation describes potassium nitrate as an implicit salt of potassium hydride and oxoazinic acid. This new citation descibes methylammonium nitrate as an implicit salt of methanamine and oxoazinic acid. The new source is a database as opposed to a chemical supplier's catalogue. I still need to know the answer to my earlier question: was my edit to nitric acid the general idea, Rifleman?--Plasmic Physics (talk) 00:15, 6 June 2010 (UTC)
- I'm not sure why you wish to expend so much effort in this direction, which IMHO, is futile. With regard to your edit to nitric acid in particular, I don't think it helps, but since our "other names" section is such a mess in general anyway, it probably does little harm. Better in that box than in the text. With regard to Ben's comment, if you're that adamant about doing the job which a computer is more suited for, please seek advise from him and PC about how to navigate IUPAC's rules to generate real IUPAC names according to the rules, not simply something you can find off the internet. Verifiability is not truth.--Rifleman 82 (talk) 03:16, 6 June 2010 (UTC)
- Oh it's no effort at all! BTW, I'm not generating the IUPAC names, I'm using a plugin from ChemAxon's Marvin. For more information about its generating ability, visit [11]. I'm not sure why Physchim reverted my edit to nitric acid, as I added oxoazinic acid to the other names section and removed it from the body of the article, just as you recommended.--Plasmic Physics (talk) 06:50, 6 June 2010 (UTC)
I don't think the database is a reliable source either.
The most appropriate source for IUPAC names is clearly IUPAC itself.
A quick Google search for "IUPAC nomenclature" gave the following relevant results:
- http://www.iupac.org/
- http://old.iupac.org/nomenclature/index.html
- http://www.chem.qmul.ac.uk/iupac/
- http://www.acdlabs.com/iupac/nomenclature/
- http://old.iupac.org/publications/books/seriestitles/nomenclature.html
- http://www.rsc.org/pdf/general/append.pdf
Try these first.
Ben (talk) 00:55, 6 June 2010 (UTC)
- That's just the thing, IUPAC itself does not provide any names, except for the purpose of proving examples of naming procedure, it is not a catalogue for names. IUPAC only provides the means of constructing approved names. These constructed names must be found elsewhere. I have the IUPAC names already, I just need proper online sources that I can cite the names.--Plasmic Physics (talk) 03:13, 6 June 2010 (UTC)
Right, so this all comes down to whether Marvin's automatic name generator is a reliable source of IUPAC names. What do you think?
Ben (talk) 08:23, 6 June 2010 (UTC)
- I can assure you that I have not intend to user Marvin's generator as a reliable source. There is no purpose if no one actually uses it, that's why I check online if anyone uses it before I decide to add it or not.--Plasmic Physics (talk) 08:28, 6 June 2010 (UTC)
That method (using Marvin for structure → name then using Google to check if name is used) is OK in principle, but you need to add a final step: discard any usage online in unreliable sources. Even then, the name might be used very little, in which case it's probably best to ignore it.
Maybe we can string together some guidelines on reliable sources for chemical nomenclature.
Ben (talk) 10:33, 6 June 2010 (UTC)
- That would be most useful, I'm honestly looking forward to continueing with my mission.--Plasmic Physics (talk) 11:20, 6 June 2010 (UTC)
- When should I be expecting a draft of the proposed guidelines?--Plasmic Physics (talk) 00:08, 8 June 2010 (UTC)
- If inserting doubtful IUPAC is your mission I think there is a problem. PubChem, ChEBI,ChemIDplus provide IUAPC names. I think there is enough places to search. Biglama (talk) 13:32, 24 June 2010 (UTC)
- Inserting doubtful names is not my intention, doubtfulness is just a common problem by coincedence. I intend to add current, systematic IUPAC names. We've established that databases are not acceptable refferences for nomenclature, so none of your proposed sources will work, but the effort is appreciated.--Plasmic Physics (talk) 00:17, 25 June 2010 (UTC)
It is clear that Plasmic Physics is still engaging is disruptive edits. If he did not already have a long history of this behaviour I would discuss it directly with him/her. However, he/she is clearly trying to provoke edit wars. Drawing his/her attention is the last thing I want to do. This would simply draw more attacks. Working around his/her behaviour is extremely time consuming and is limiting my productivity. Please, please, please block his/her URL. Azidothymizeal (talk) 03:02, 21 September 2010 (UTC)
New categories such as Category:Silanebis(olates). This new category is extremely strange and very confusing. Where is the logic behind it ? It seems not to be advised by IUPAC. I could not find any information on it by browsing the web with Google. Google most often refers to this page. Moreover, it increases the granularity level of the information in a useless way. I do not think it makes sense. It would be better inspired to keep information simple and straightforward (KISS principle) and to avoid to make things too complex. Remember the citation of Albert Einstein: "As simple as possible, but not simpler than needed". Best regards, Shinkolobwe (talk) 18:13, 21 November 2010 (UTC)
- For more information on the nomenclature of inorganic silicates, look at pp. 139-140 of Red_Book_2005.pdf http://old.iupac.org/publications/books/rbook/Red_Book_2005.pdf; Best regards, Shinkolobwe (talk) 18:37, 21 November 2010 (UTC)
Category:Silanuides: this very uncommon chemical category does not refer to a widely adopted IUPAC convention but to an obscure website selling chemicals: http://www.molport.com/
http://www.molport.com/buy-chemicals/moleculelink/disodium-hexafluorosilanediuide/158492
This website comprises most of the few results (only 10!) on the topic given by a Google search.
This kind of category makes no sense and should be avoided because it can only trow confusion in the mind of the readers. Shinkolobwe (talk) 21:33, 21 November 2010 (UTC)
{{tl|Hydrogen compounds}}[edit]
{{Hydrogen compounds}} Better renamed hydrogen acids? --Rifleman 82 (talk) 04:46, 10 October 2010 (UTC)
- Hydrogen compounds include acids, and therefore, I think we should either continue using "Hydrogen compounds" only, or split into "Hydrogen compounds" (e.g. Hydrogen fluoride, hydrogen sulfide) and "Hydrogen acids" (e.g. Hydrochloric acid, molybdic acid). What do you think? YOSF0113 (talk - contributions) 04:50, 10 October 2010 (UTC)
When you look at it from an absolutist perspective, any hydrogen-containing compound can act as a Bronsted acid. You just need to find a sufficiently strong base, and indeed we do have very strong bases (see Bordwell's pKa tables, though I'm not sure if all the values are determined experimentally). To take this list to another logical conclusion, we would need to include almost all organic compounds (if we define organic chemistry as chemistry of the C-H bond).
Just looking at the title, I would imagine such a list would include all the primitve -anes, e.g. alane, gallane, methane, oxidane (OH2), azane (NH3), etc. --Rifleman 82 (talk) 05:46, 10 October 2010 (UTC)
- I do not see the point of the category, as Rifleman points out, virtually all compounds containing H can be deprotonated, so all are from some perspective acids. But I dont pay much attention to categories, so I am no expert. --Smokefoot (talk) 14:04, 10 October 2010 (UTC)
- It seems to me that a prerequisite for deciding if templates and categories are useful, or what they should be named, is deciding what they are supposed to cover. At present, it seems to me that the template is under-populated for most definitions. It includes sulfamic acid which exists predominantly as the zwitterion +H3N–SO3−... so why not glycine and the other amino acids? The category "hydrogen compounds" uses the definition "Hydrogen compounds are those chemical compounds which contain hydrogen" which covers literally millions of additional compounds that are not on the template, and hydrogen acids as a title is similarly problematic as most hydrogen compounds can be deprotonated. Further, the metal hydrides (like sodium hydride) clearly don't belong in "hydrogen acids", but they are in the category metal hydrides which is a sub-category of "hydrides" which is itself a sub-category of "hydrogen compounds", so a rename would put a metal hydrides category as a daughter category of hydrogen acids - clealy a problem. Alternatively, if we had a hydrogen acids template but a hydrogen compounds category, we run into wondering whether we need a hydrogen acids category and also how they all differ. There are also oodles of ionic compounds containing hydrogen, many of them (like ammonium sulfate) acidic in aqueous solution, and so definite candidates for "hydrogen acids" (and also for "hydrogen compounds", by the way). I really think the first question needs to be the definitions to govern these categoroies and templates, and only then can we sensibly discuss populating them. EdChem (talk) 14:40, 10 October 2010 (UTC)
- YOSF0113 is going to try to help whether the help is needed or not. That is the style of this editor. We do not want this tagging on all compounds that contain H (hydrates?, hydrocarbons?) and his categorization should stop. He could be encouraged to be more consultative.--Smokefoot (talk) 13:26, 11 October 2010 (UTC)
- A category containing every compound which possesses a hydrogen atom, whilst implied by the definition I quoted above, is certainly not something I would advocate as useful. Just to be clear, I was commenting on the definitional issue raised by these teamplates and categories, not advocating for a definition nor suggesting any flurry of activity was warranted. EdChem (talk) 13:49, 11 October 2010 (UTC)
- YOSF0113 is going to try to help whether the help is needed or not. That is the style of this editor. We do not want this tagging on all compounds that contain H (hydrates?, hydrocarbons?) and his categorization should stop. He could be encouraged to be more consultative.--Smokefoot (talk) 13:26, 11 October 2010 (UTC)
- It seems to me that a prerequisite for deciding if templates and categories are useful, or what they should be named, is deciding what they are supposed to cover. At present, it seems to me that the template is under-populated for most definitions. It includes sulfamic acid which exists predominantly as the zwitterion +H3N–SO3−... so why not glycine and the other amino acids? The category "hydrogen compounds" uses the definition "Hydrogen compounds are those chemical compounds which contain hydrogen" which covers literally millions of additional compounds that are not on the template, and hydrogen acids as a title is similarly problematic as most hydrogen compounds can be deprotonated. Further, the metal hydrides (like sodium hydride) clearly don't belong in "hydrogen acids", but they are in the category metal hydrides which is a sub-category of "hydrides" which is itself a sub-category of "hydrogen compounds", so a rename would put a metal hydrides category as a daughter category of hydrogen acids - clealy a problem. Alternatively, if we had a hydrogen acids template but a hydrogen compounds category, we run into wondering whether we need a hydrogen acids category and also how they all differ. There are also oodles of ionic compounds containing hydrogen, many of them (like ammonium sulfate) acidic in aqueous solution, and so definite candidates for "hydrogen acids" (and also for "hydrogen compounds", by the way). I really think the first question needs to be the definitions to govern these categoroies and templates, and only then can we sensibly discuss populating them. EdChem (talk) 14:40, 10 October 2010 (UTC)
- i would get rid of all oxyacids —Preceding unsigned comment added by 170.170.59.138 (talk) 18:33, 19 October 2010 (UTC)
So... how should we deal with this? Should we remove this arbitrary and unhelpful template? --Rifleman 82 (talk) 08:28, 10 December 2010 (UTC)
- I think so, who needs it?
Reorganization of Category:Silicon compounds cattree[edit]
Notice: There is an ongoing discussion on the categorization of silicon compounds on User talk:Plasmic Physics#Reorganization of Category:Silicon compounds cattree. If anyone is interested in, (s)he is invited to participate. --Leyo 10:21, 3 November 2010 (UTC)
New categories such as Category:Silanebis(olates). This new category is extremely strange and very confusing. Where is the logic behind it ? It seems not to be advised by IUPAC. I could not find any information on it by browsing the web with Google. Google most often refers to this page. Moreover, it increases the granularity level of the information in a useless way. I do not think it makes sense. It would be better inspired to keep information simple and straightforward (KISS principle) and to avoid to make things too complex. Remember the citation of Albert Einstein: "As simple as possible, but not simpler than needed". Best regards, Shinkolobwe (talk) 22:21, 21 November 2010 (UTC)
- For more information on the nomenclature of inorganic silicates, look at pp. 139-140 of Red_Book_2005.pdf http://old.iupac.org/publications/books/rbook/Red_Book_2005.pdf; Best regards, Shinkolobwe (talk) 22:21, 21 November 2010 (UTC)
Category:Silanuides: this very uncommon chemical category does not refer to a widely adopted IUPAC convention but to an obscure website selling chemicals: http://www.molport.com/
http://www.molport.com/buy-chemicals/moleculelink/disodium-hexafluorosilanediuide/158492
This website comprises most of the few results (only 10!) on the topic given by a Google search.
This kind of category makes no sense and should be avoided because it can only trow confusion in the mind of the readers. Shinkolobwe (talk) 22:21, 21 November 2010 (UTC)
Verification[edit]
The verification process, in which we try to verify the data displayed in the chembox (more information here), is making quite some progress. We started off months ago verifying all the CASNo's, but that has stalled now (partially due to manpower, partially due to not many more pages being available on commonchemistry, which makes verification of CASNo's more difficult).
In the meantime we made contact with the FDA, who have given us a long list of UNIIs (Unique Ingredient Identifiers), which I have since (on the basis of the verified CASNo's) incorporated into our articles (note: not all compounds have a UNII, for those the field is verified to be blank - it is still a verified value).
ChemSpider provided us with a large list of pages for which the ChemSpiderID was verified, and some have noticed the long run of script assisted edits in which I updated the CSID's. Another set was done by hand and those were done in the same run. This week I made a checklist of compounds which did not have a verified CSID but did have a verified CASNo, and I am pleased to announce that I now verified for all of those the CSIDs.
Hence, CheMoBot now follows CAS numbers, ChemSpiderIDs and UNII (note: only the first CASNo, UNII and ChemSpiderID are verified and (should) point to the correct isomer/composition&c., the others are, at the moment, not included in the process, they may be flagged but not via CheMoBot). This now enables to semi-automatically fill the StdInChI and StdInChIKey fields from ChemSpider, which then really enables image verification and verification of physical data (within the limits of the accuracy of physical data) as we really know what compound the page is talking about. When all the StdInChIs and StdInChIKeys are set for the verified compounds, I will also make the bot follow those appropriately.
To put it in numbers: 2571 of 6815 pages with a chembox are verified for CAS, UNII and CSID (37.7%); 1227 of 4697 pages with a drugbox (26.1%). Our list of ChemSpiderIDs is 7459 items long (but that contains 'duplication').
I am making this announcement here, so I get a wider public: please, when you encounter pages which have all three correct, but which have not been indexed in Wikipedia:WikiProject Chemicals/Index or Wikipedia:WikiProject Pharmacology/Index, then add a revid with the correct values to the appropriate index (manuals there). The more we get there, the more correct StdInChI's we can get, and the more data we can properly verify to the correct compounds. Also, there may be cases where there are mistakes, I would be pleased if you would either point me to them, or repair them (and update the index as needed). CheMoBot will do the rest (in terms of notifying you of unverified data). It would of course be our goal to get to a near 100% coverage in the end, and you can be of help there. Thanks! --Dirk Beetstra T C 10:11, 12 November 2010 (UTC)
CheMoBot autoconfirmed[edit]
I just had to confirm an edit by CheMoBot ("Accept: require "autoconfirmed") to taurine. Is there a rreason that CheMoBot does not have autoconfirmed status? Cacycle (talk) 07:31, 15 November 2010 (UTC)
- I'm always confused. This means that if the edit before is confirmed, then CheMoBot's edit is also confirmed, right. If the edit before is not confirmed, then this does not confirm the edit either, right. If so, then there is no reason why CheMoBot does not have it. --Dirk Beetstra T C 11:00, 15 November 2010 (UTC)
user:Plasmic Physics (continued from above)[edit]
New categories such as Category:Silanebis(olates). This new category is extremely strange and very confusing. Where is the logic behind it ? It seems not to be advised by IUPAC. I could not find any information on it by browsing the web with Google. Google most often refers to this page. Moreover, it increases the granularity level of the information in a useless way. I do not think it makes sense. It would be better inspired to keep information simple and straightforward (KISS principle) and to avoid to make things too complex. Remember the citation of Albert Einstein: "As simple as possible, but not simpler than needed". Best regards, Shinkolobwe (talk) 18:13, 21 November 2010 (UTC)
- For more information on the nomenclature of inorganic silicates, look at pp. 139-140 of Red_Book_2005.pdf http://old.iupac.org/publications/books/rbook/Red_Book_2005.pdf; Best regards, Shinkolobwe (talk) 18:37, 21 November 2010 (UTC)
- It appears to be that there are quite a few unnecessary categories among the organosilanes. Some only have two or fewer articles in them. When many of them have few articles in them, the usefulness of the categorization is lost. ChemNerd (talk) 01:03, 22 November 2010 (UTC)
- Concur. The whole point is to help group related articles, not create a complete multilevel ontology or just every possible or most specific cat one can think up. Category:Group 4 silicates? DMacks (talk) 10:53, 23 November 2010 (UTC)
- It appears to be that there are quite a few unnecessary categories among the organosilanes. Some only have two or fewer articles in them. When many of them have few articles in them, the usefulness of the categorization is lost. ChemNerd (talk) 01:03, 22 November 2010 (UTC)
Excessive number of chemical identifiers[edit]
I'm concerned about the excessive number of chemical identifiers that are creeping into so many chemistry articles. Dimethyl sulfoxide, for example, has six links to PubChem. If there is an isotopically labeled version notable enough to be mentioned in the article, maybe it's worth a link to that one ... but really, who could possibly need this many links to PubChem listings of DMSO with various combinations of isotopes? Sorry for the venting, but could we use a little common sense? ChemNerd (talk) 00:57, 22 November 2010 (UTC)
- Agree Excessive chemical identifiers can interfere with readability. At least ordinarily. The chemistry is complicated enough without dredging up these obscure isotopomers (and for DMSO, many permutations are possible). Maybe we should create a new statement of what Wikipedia is not, not Chemical Abstracts and not Beilstein. But maybe others have alternative views/suggestions. The topic opens many questions. --Smokefoot (talk) 01:59, 22 November 2010 (UTC)
- Adding labeled isotopes is maniacal and excessive. If User:Plasmic Physics continues (See also #True pyrrole? and other topics here and User_talk:Plasmic_Physics#Glucose) may be only a block will do.--Wickey-nl (talk) 10:13, 23 November 2010 (UTC)
- Hmmm .. I did argue that isotopomers might want the identifier in the chembox, but did not realise that that might here and there lead to excessive situations. For the 'common' NMR solvents the common 2H (and maybe 13C) varieties might be of interest (as some of these database links link through to a wealth of information), but it should stop there I think. --Dirk Beetstra T C 10:35, 23 November 2010 (UTC)
- Well, I am not sure. I, for one, am certainly not only looking for the formula. Physical data is also something that I am looking for on a regular basis. And to check it, I then often go through the identifiers.
- I can agree, most people do not look at the chembox at all, but may read the text. That is why it is moved to the side. Should it have all possible data .. well, if someone is looking for a certain thing, then that is certainly something that should be there. We are not to decide what information is hardly useful - if someone needs it, it is useful to be there. --Dirk Beetstra T C 11:50, 23 November 2010 (UTC)
- If someone is looking for data for professional use he will certainly not depend on Wikipedia. It is usefull, but do not overestimate--Wickey-nl (talk) 12:27, 23 November 2010 (UTC)
- I did not say that I depend on it .. being unreliable does not mean untrue (and that is why we are now so hard working to get at least the identifiers verified - so it is quicker to check whether the data is correct). --Dirk Beetstra T C 12:47, 23 November 2010 (UTC)
- If someone is looking for data for professional use he will certainly not depend on Wikipedia. It is usefull, but do not overestimate--Wickey-nl (talk) 12:27, 23 November 2010 (UTC)
For what it is worth, I have started to remove isotopologues and other uncommon compounds (unusual/less common hydrates, e.g.) from the list of identifiers. If an isotopologue is of reasonable interest (and not worthy an own article) then it could be here, but others should go. Same goes for hydrates, MgSO4 has interesting hydrates (but not all!), the hydrate of DMSO is not of particular interest. --Dirk Beetstra T C 12:03, 23 November 2010 (UTC)
Long chemboxes ...[edit]
User:Wickey-nl noted that some chemboxes are extremely long, sometimes significantly longer than the pagetext (I could argue now, go expand the article, there is enough data to talk about, but I will not).
A solution to those long chemboxes would be to have a possibility to collapse the modules. I have now changed {{Chembox Identifiers/sandbox}} so that it is standard still normal (but it has a hide button), and with a parameter 'collapsed=yes' can be set to be collapsed standard (but then it has a 'show' button). A working example with a fake chembox is in User:Beetstra/test. It comes, until now, with a small drawback .. the modules get a slightly thicker skin. I am still tweaking to get rid of that, but if someone knows, please implement it where appropriate.
Is there any support for implementing this? --Dirk Beetstra T C 16:02, 23 November 2010 (UTC)
Nice. --Rifleman 82 (talk) 16:17, 23 November 2010 (UTC)
- Nice to me too. The question is whether these fancier ChemBoxes are more complicated to implement. We dont want to inhibit the addition of content.--Smokefoot (talk) 18:26, 23 November 2010 (UTC)
- In editmode nothing changes, the displayed code does not change, only the display is slightly different. You may be right in that if they don't see a missing InChI because the identifiers are collapsed, that they might not add it... --Dirk Beetstra T C 18:57, 23 November 2010 (UTC)
- Without doubt, Beetstra spent already a lot of time on separate buttons for the sub sections, but one button for expanding and collapsing all at once will be more convenient. Problably also with a simpler code and even with a shorter box.--Wickey-nl (talk) 11:59, 24 November 2010 (UTC)
- Actually not, this is easier to program then collapsing all modules at once. --Dirk Beetstra T C 12:06, 24 November 2010 (UTC)
- Without doubt, Beetstra spent already a lot of time on separate buttons for the sub sections, but one button for expanding and collapsing all at once will be more convenient. Problably also with a simpler code and even with a shorter box.--Wickey-nl (talk) 11:59, 24 November 2010 (UTC)
I propose to use the collapsed chembox now and see (or see not) the reactions. The layout of the pages will really improve very, very much.--Wickey-nl (talk) 13:44, 4 December 2010 (UTC)
Discussion has been revived at Wikipedia talk:WikiProject Chemistry#Chembox. DMacks (talk) 16:24, 24 January 2011 (UTC)
- That discussion has been archived into Wikipedia talk:WikiProject Chemistry/Archive 22#Chembox. -Mardus (talk) 04:28, 16 May 2012 (UTC)
Hi all, there is currently a dispute about whether WP:ALUM applies to the vitreous enamel page. The discussion is at Talk:Vitreous enamel#English variations - again. I would grateful for any input. Thanks! Wizard191 (talk) 19:21, 23 November 2010 (UTC)
Categories: Arsenic[edit]
I encourage others to examine the current categorization work in arsenic chemistry? As2S3, a polymer, and C6H4(AsMe2)2, which in my world is a diarsine, are both under Category:Monoarsanes. It just seems to me that there should be some effort to achieve consensus on broadly impacting editing to ensure that the classifications best serve readers. We decided, I think, some time ago that R3P is a phosphine, not an phosphane in our pages. So there is also the -ane vs ine issue.--Smokefoot (talk) 11:49, 24 November 2010 (UTC)
- PlasmaPhysics's editing seems to be taking up a lot of our time and energy, yet doesn't really add much. I wish he'd just stop. Or at least only do things we've all agreed on in advance. It's really annoying.
- If there's a developed WP:CONSENSUS that his categorization is problematic (either scientifically bogus, or making information/related articles less easy to find, or nonstandard terminology, or whatever), he must stop. He can do it the wikipedia way, by joining collaborative editing and asking for help beforehand, or be blocked if we need to go that far. "Consuming lots of other editors' time with no gain for WP (and loss because others need to spend time cleaning up)" is a pretty bad problem (guideline: WP:DISRUPTIVE, associated essay: WP:COMPETENCE). DMacks (talk) 12:22, 24 November 2010 (UTC)
- I've asked Plasmic Physics to joint this discussion, and to consider to follow the suggestions given. I agree that it would be good if these large scale changes to categorisation, and the data incorporation in the chemboxes needs to be discussed thoroughly before proceeding. The edits are controversial. --Dirk Beetstra T C 12:28, 24 November 2010 (UTC)
- A diarsine would suggest two arsenic atoms bonded together, monoarsane represents a derivative of singlur arsenic atom. Monoarsane does not mean that the structural formula contains one arsenic atom, it means that the structural formula contains at least one arsenic centre.
- On the -ane vs -ine, issue: that is not my concern. I do not own the rights to any one category, if the name bothers you that much, then change it, as long as it still represents the same structure. The compound categories are unorganised, and since no one else is taking charge, I'm busy attempting to group structurally similiar compounds together. I do this following recommended IUPAC nomenclature of chemical moieties. In some circumstances, my categories combines at least two other categories used on a particular article, saving space. While I have to admit I practised on silicon, I have pretty much sorted. It is finished for now at least, there are a couple of empty categories awaiting speedy deletion. Other than trimming a few empty categories, it is complete. Plasmic Physics (talk) 13:18, 24 November 2010 (UTC)
Plasma, you keep relying on your own fixed ideas of the way names should be (diarsines have As-As bonds for example), but you are usually pushing an agenda that does not jive with ordinary readers. Yes, I know that diarsines are supposed to have As-As bonds, and probably in some part of IUPAC, As2S3 is an arsane. But the chemist's language is not as logical as the one you are trying to impose. In my opinion your edits are of negative value and your underperformance consumes editing time that could be be fruitfully applied to helping our readership. --Smokefoot (talk) 14:08, 24 November 2010 (UTC)
- (ec)Saving space in a cat-list is about the worst possible reason I can think of to combine cats. The goal is to help readers find related articles. You seem to be saying that if an article sits at the intersection of two classifications, we should force readers to look first at that intersection-cat and then branch out to the two ancestors? See WP:OC#NARROW and WP:OC#SMALL. How is Category:Organoarsenic sulfides useful? From the only article in it, gotta navigate up two levels just to find any related article, and then maybe drill down to find more. And when all that clicking around is done, there are only 16 articles in the whole of Category:Organoarsenic chalcogenides and its subcats...hardly unwieldy to have them all together! DMacks (talk) 14:11, 24 November 2010 (UTC)
- The Arsenic categories which I have created are not fully filled, there are more candidates for Category:Organoarsenic sulfides then are currently listed. It takes time to manually categorise articles, a process that interupted by this discussion. At least all the articles in Category:Organoarsenic chalcogenides not in Category:Organoarsenic oxides should be categorized in Category:Organoarsenic sulfides. Plasmic Physics (talk) 23:12, 24 November 2010 (UTC)
- WP:OC#SMALL is not applicable here, there is a large potential for growth. Wikipedia does not have aticles on every compound already discovered and documented. Plasmic Physics (talk) 23:28, 24 November 2010 (UTC)
- Plasmic Physics, WP:OC#SMALL is partially applicable - there are only so many arsenic compounds sufficiently notable to be included. Although there are a plethora of arsenic compounds that in the end would fit into a category, it does not make sense to now have 15 categories, 10 of them having just 1 compound in it. It makes the compounds practically impossible to find. It makes sense if there are categories with, say, 50 compounds - then you would start to split them up one level, and when those new sub-levels start growing too much you do a next one. Please be careful with this. --Dirk Beetstra T C 08:27, 25 November 2010 (UTC)
- Please point out at least two other three two arsenic related categories containing only one compound in it, which I created. Category:Organoarsenic sulfides does not count as I already mentioned the reason for it only having one compound. Plasmic Physics (talk) 08:43, 25 November 2010 (UTC)
- Oh sigh. 1, 2 .. less than 5 .. silicon compounds in a silicon cat, sulfur compounds in a sulfur cat, and compounds with both a silicon and a sulfur in both the silicon cat ánd in the sulfur cat, not in a silicon-sulfur-cat. What is next .. a silicon-sulfur-oxygen-cat, and then a silicon-sulfur-oxygen-nitrogen-cat? Because that is where, according to your catting, Arsthinol is going to be? --Dirk Beetstra T C 11:20, 25 November 2010 (UTC)
- I have not observed any such minimum limit, as some categories not created by me, often has only one participent. Plasmic Physics (talk) 22:52, 25 November 2010 (UTC)
- Category:Phosphoroamidethioates is a prime example of category too specific for even me, not created by me. What about Category:Thiuram disulfides. Plasmic Physics (talk) 23:26, 25 November 2010 (UTC)
I changed the categorization of some organoarsenice compounds. These changes remove or simplify categorization of those implemented by PlasmaPhysics. To avoid some sort of revert struggle, I request guidance on whether or how to continue. I think that organoarsenic is sufficiently narrow. For myself, that is a useful grouping. --Smokefoot (talk) 14:21, 26 November 2010 (UTC)
- I find it highly annoying when discussions like this are just abandoned, users just leave complaints, but solutions are rare, and even when solutions are suggested, they are not seriously considered. This has happen several times over, and is not helpful in developing guidelines for Wikipedia. Plasmic Physics (talk) 19:39, 27 November 2010 (UTC)
The problem with conversations being dropped just reflects the irregular attention spans of this collection of volunteers. I agree that we should write a style guide for the categories. --Smokefoot (talk) 00:28, 28 November 2010 (UTC)
- I have momentarily stopped creating new categories, but now a few select number of users are impulsively emptying categories. I ask that rather we discuss it fits in the talk pages, on a per category basis. If everyone just goes and impulisively empties a category when they have an opinion, then we will have a mess. Plasmic Physics (talk) 00:32, 29 November 2010 (UTC)
- Indeed, discussions sometimes stop as the volunteers have more important things on their mind. I also agree that that can be irritating.
- There are problems with the categorisation, Plasmic Physics showed two which are just a step too far: Category:Phosphoroamidethioates and Category:Thiuram disulfides. I would suggest a 'elemental' tree:
- Phosphorus compounds, which might be split by oxidation state or valency, and then by P1, P2, maybe P3, and 'poly'-phosphorus (n>2 or 3).
- For Nitrogen I would not bother splitting by oxidation state but rather immedately by number of nitrogens in the product.
- Of course, this is going to give problems with 'mixed oxidation state' compounds etc, but they can, for phosphorus, e.g. be grouped in a 'mixed-oxidation-state' / 'mixed-valency' cat, which then might be split further. I would strongly advice against categories of the type 'containing both a nitrogen and an oxygen', those would be in the appropriate nitrogen cats, ánd be in the appropriate oxygen cats.
- Next to this tree, there should be a functional group-tree: phosphates, phosphines, etc.
- Would this be useful as a start, or do I already miss cases which are going to be problematic here? --Dirk Beetstra T C 09:52, 29 November 2010 (UTC)
- This sound like a start. The elemental tree that I use is as follows: valency, chain length, functional group, number of functional group. The organo category: the more electropositive element first, and the two elements must be directly bonded, no more than two elements for category. Organosilicon oxides is an example, not organooxygen silicides or organosilicon nitride oxides, and it cannot include a compound like (trimethylsilyl)methanol, as there are no silicon oxygen bonds. This is the core tree structure that I have developed after studying the carbon category tree, and an assortment of other element category trees. Besides this core tree, other miscelaneous sub-categories may be created as appropriate. I don't like being to specific, I prefer to create general class categories, like halomonosilanes. I don't start off with chloromonosilanes, what am going to do when I have to categorise tetrafluorosilane? (rhetoric). I only specify when I feel there are enough candidates, and when I see potential growth in the near future. This is a different approach from other users, who specify on the first category. Plasmic Physics (talk) 10:55, 29 November 2010 (UTC)
- I agree with the idea of having a general pattern to use when creating levels of detail for the cats, but I strongly disagree with the idea of actually implementing it across-the-board. We need to think about what the useful groupings are, not just how to narrow down a large group, and not just to create tons of "X+Y" intersection cats. Think about going to a library or chemical reference; you have a compound that you know about and want to find a list of similar ones. Are you likely to say "mine has a propyl group, I'd like to see others with propyl groups" (your use of "chain length" as a second-level hierarchy)? DMacks (talk) 13:32, 29 November 2010 (UTC)
- That depends whether propyl is the determining group - if a more common functional group is present, it overides propyl - if there is also a butyl group present then only it's corresponding category is selected and propyl will be omitted. Note propyl is a bad example, as it does not conform ideally to my suggested tree. Plasmic Physics (talk) 14:10, 29 November 2010 (UTC)
I, Benjah-bmm27, and Wickey-nl, three two active editors in this space, have made our statements above, in effect: we recommend against any categorization work by PlasmaPhysics.--Smokefoot (talk) 13:55, 29 November 2010 (UTC)
- I did not mention it in that way, indeed. Lets us first come up with a better categorisation scheme (and agree on that), before implementing it. --Dirk Beetstra T C 14:07, 29 November 2010 (UTC)
Prediction: PlasmaPhysics will to push the boundaries of common sense (the editor that brought you "Silanuides"). Eventually some other editor will point out new abuses, then these pages will again be host to hand-wringing discussion like this one. Check out (i) this page for the amount of time and energy diverted to accommodating and corralling this editor and (ii) then check out User talk:Plasmic Physics. I just hope that those that are appeasing this editor will follow through with policing the work. --Smokefoot (talk) 19:01, 29 November 2010 (UTC)
- What's wrong with silanuides? Using the Red Book, either additive or substituive nameing is allowed. Plasmic Physics (talk) 22:56, 29 November 2010 (UTC)
- For what it is worth, I have reverted most of the re-categorisations of today to silicon compounds. I again suggest: first come up with a recategorisation, and then implement it. Plasmic Physics, many people do not agree with what you are doing, I was hoping that you would consider to come up with a better scheme, discuss it, and then maybe help implementing it, but I am now shifting towards considering that you should not be doing any re-categorisations anymore, and I am even afraid that we will have to check all non-systematic categorisations that you perform(ed). Sigh. --Dirk Beetstra T C 09:26, 30 November 2010 (UTC)
Anemonsäure[edit]
Does anyone know the English name of Anemoninsäure (=German name) (HOOC-CH=CH-CO-CH2-CH2-CO-CH2-CH2-COOH, SMILES: OC(=O)\C=C\C(=O)CCC(=O)CCC(=O)O, InChIKey: VWRXCXNVGUBJFZ-HWKANZROSA-N)? Anemonic acid? Thanks, ἀνυπόδητος (talk) 12:55, 26 November 2010 (UTC)
And what about Anemonsäure (SMILES: OC(=O)\C=C\C=1CCC(=O)C=1CC(O)=O, InChIKey: BKJWYNNXFMEABE-DUXPYHPUSA-N)? --ἀνυπόδητος (talk) 15:05, 26 November 2010 (UTC)
- Anemonic acid would be the obvious translation, but it appears to be used for a sesquiterpinoid (C15H14O7), not the monoterpinoid that you describe by your SMILES. Anenomin (C15H12O6) has also been used as a name, but for a different compound. Ref: [12] and Google. Physchim62 (talk) 15:32, 26 November 2010 (UTC)
- I can't access your source. Is there actually something called anenomin? Anemonin is C10H8O4 (see below). --ἀνυπόδητος (talk) 11:49, 27 November 2010 (UTC)
- Anemonic acid would be the obvious translation, but it appears to be used for a sesquiterpinoid (C15H14O7), not the monoterpinoid that you describe by your SMILES. Anenomin (C15H12O6) has also been used as a name, but for a different compound. Ref: [12] and Google. Physchim62 (talk) 15:32, 26 November 2010 (UTC)
- Roscoe-Schorlemmer's Lehrbuch der organischen Chemie. gives a good clue where it comes from and page 4 is most likely the mechanism of from Anemonin to Anemonin acid.--Stone (talk) 17:10, 26 November 2010 (UTC)
- Nice sources. Do you mean that Anemoninsäure (the one with the linear C10 framework) = Anemonin acid, or was that just an ad-hoc translation? --ἀνυπόδητος (talk) 11:49, 27 November 2010 (UTC)
The article is saying about its basicity being moderate in one section and strong in another. Google books are divided on this - some say strong some say weak base. Could someone rectify this in the article, as anons keep changing it either way. Thanks. Materialscientist (talk) 09:30, 27 November 2010 (UTC)
- Ammonia is a weak base, pKb = 4.76 (from memory). Its conjugate base, the amide ion (NH2−), is a strong base. Its conjugate acid, the ammonium ion (NH4+), is a weak acid, pKa = 9.24. I suspect that Google books that are unclear on this point are badly written. For example, it is not uncommon to find pKa = 9.24 quoted for ammonia, but it actually is the pKa of the ammonium ion; the actual pKa for ammonia is around 30 (again from memory). EdChem (talk) 09:49, 27 November 2010 (UTC)
New user wants WP:FEEDback[edit]
A new editor has created Barton Decarboxylation and has requested feedback at Wikipedia:Requests for feedback/2010 November 30#Barton_decarboxylation. If someone here would please take a look, I'd appreciate it. Thanks, WhatamIdoing (talk) 02:24, 1 December 2010 (UTC)
Condensed formulas in Chembox?[edit]
I think it will be good to put condensed formulas in the Chembox. After images and trivial names, condensed formulas are worldwide most used to recognize compounds and to refer to it. Think of magnesium nitrate Mg(NO3)2, propionic acid CH3CH2COOH, phenol C6H5OH, etc. It should not replace the simple molecular formula. For bigger compounds the field can be omitted or a semi- condensed formula can be used or the shortest molecular formula form can be used (repeated). The best place would be between the images and the IUPAC name.--Wickey-nl (talk) 13:36, 4 December 2010 (UTC)
- I have thought about that too, but I think that the current system is good one. For organic things, there are many ways to write formulas and we'd have too much confusion. For the poor inorganic folks, the formula Mg(NO3)2 of course does not convey essential information, i.e. that there is no molecule of this stuff - it is a 3-d polymer that disintegrates instantly in solution. "(Mg(NO3)2)n" seems too complicated? And so it goes. I do agree that more descriptive formulas should be in the opening paragraph (not empirical formula), where we have the opportunity to discuss the meaning of the formula. --Smokefoot (talk) 13:59, 4 December 2010 (UTC)
Controversial use of the term ‘Oxoazinic acid’ in the chembox of Nitric acid[edit]
I propose to totally omit the term ‘oxoazinic acid’ from the chembox of nitric acid because it seems mostly to be used by spammers or crasy websites supposed to sell chemicals and never by honorable chemical companies such as, e.g., Sigma Aldrich or Merck.
Before adding obscure, or worse, inexact, nomenclature terms to Wikipedia, it is necessary to pay attention to the impact it might have in the real world. Wikipedia is more and more renowned in the field of science and techniques and increasingly used by students or professionals in various fields. Often now, Wikipedia behaves like a resonance box or an amplification system and is also frequently wildly recopied by other websites for unknown purposes. According an inadequate importance to obscure terms such as 'oxoazinic acid' and worse, promoting them as “SystematicName” or “IUPACName” in chemboxes could encourage their use worldwide and in a few years, it could be extremely difficult, if not impossible, to get rid of an erroneous and embarrassing nomenclature. As for a viral infection, a bad habit should be immediately stopped in the egg and no chance left to an ugly contamination to propagate and to develop into a pandemia.
Today, I resumed again my search attempts to collect valuable information about the term 'oxoazinic acid'. Hereafter, the very disappointing results returned by three well established search engines:
- Google: only 245 results (most of them from http://www.molport.com or from spammers sites trying to sell chemicals !);
- Google Scholar: only 1 results !
- ScienceDirect: only 1 results: Article in Press, Corrected Proof, not yet published:[1]
“A new approach to predict the strength of high energy materials” Original Research Article. Journal of Hazardous Materials, In Press, Corrected Proof, Available online 2 November 2010. Mohammad Hossein Keshavarz, Mohammad Ghorbanifaraz, Hadi Rahimi, Mehdi Rahmani. Department of Chemistry, Malek-ashtar University of Technology, Shahin-shahr, P.O. Box 83145/115, Islamic Republic of Iran.
From the abstract of this paper, it is not possible to verify the claim proposed by Plasmic Physics because the term 'oxoazinic acid' does not appear in the abstract itself, nor in the title of the paper. The term 'oxoazinic acid' could be attributable to any of the functional groups associated to the compounds cited in the abstract: nitroaromatics, acyclic and cyclic nitramines, nitrate esters and nitroaliphatics.
Ordering the paper costs US $ 41.95 and could causes notable troubles to the person making the order because of the delicate character of the subject treated and the possible negative implications in the daily life. So, in the practice, the information about 'oxoazinic acid' presumably given in the paper cannot be easily verified and such a reference does not fulfill its basic objective, nor confirm anything.
Adding an isolated and obscure reference from a paper even not yet published does not constitute a proof or a valuable argument to demonstrate a doubtful terminology.
This is why, I propose to also remove this reference from the main page of nitric acid.
The website of http://www.molport.com also contains unreliable information on strange chemical compounds. One of my most surprising finding was the following one:
Oxoazinic acid plutonium: HNO3Pu
http://www.molport.com/buy-chemicals/moleculelink/about-this-molecule/6107333
Not only, this approximate term does not reflect the very complex chemistry of plutonium, a redox-sensitive radionuclides with multiple valences, but the molport company even proposes to sell it ! As plutonium is a strategic fissile material (and also a highly radiotoxic alpha emitter), its use is severely restricted to avoid the proliferation of nuclear weapons. International treaties and IAEA safeguard standards severely limit plutonium uses and dissemination. It is highly improbable that the molport company should be authorized to sell such a substance. So, I question the content and the value of this website often used by Plasmic Physics to support strange nomenclature rules.
All that, simply to say that referring to websites such as http://www.molport.com/ or http://www.chemaxon.com/ for systematic nomenclature purposes can be problematic. Such sites can be useful to draw chemical structures but must always be used with discernment and critical mind. Best regards, Shinkolobwe (talk) 18:09, 4 December 2010 (UTC)
- I would like to endorse your idea to remove such a name. One can also note that the molport page has links to other sites that only use the term nitrate. As one who works for a catalogue company (Alfa) - we also only use the term nitric acid. Also since nitric acid has its own UN number (UN2031), then the likelihood of change is much more remote. Ronhjones (Talk) 23:03, 4 December 2010 (UTC)
- I never use Molport, Sigma Aldrich or Chemaxon for my references. In a earlier discussion we established that these are not appropriate references. The reference I gave in the nitric acid page conforms to the guidelines, despite the source not being publically available, this is allowed as per the relevant wiki template page. The source itself, references a book: Homburg, Explosives (sixth ed.), Wiley-VCH (2007), for the term oxoazinic acid. More over, oxoazinic acid fully complies with substitutive IUPAC nomenclature as per the red book. Plasmic Physics (talk) 01:29, 5 December 2010 (UTC)
- ..., "oxoazinic acid fully complies with substitutive IUPAC nomenclature as per the red book", ... .
I spent many time in the IUPAC red book and I did a search for the term oxoazinic in the PDF file of the red book: I could not find it. Hydroxidodioxidonitrogen – which is proposed as nomenclature name – has the merit to be naturally explicit and directly understandable by everyone. Who does intuitively know the substitutive IUPAC nomenclature ? Moreover, the carbocentrism has its limits in nomenclature, and I think that many inorganic chemists and geochemists will never follow uselessly complex rules in their activity fields. Nomenclature is not a cerebral sport: its main objective when choosing a name is to avoid any ambiguity and to be unequivocal in the description of the chemical substance. At the contrary, the term oxoazinic acid is very equivocal and practically not used. The reference to the not yet published paper on explosives is the only one I could find with Google Scholar and ScienceDirect: this clearly attests the rarity of this term. Shinkolobwe (talk) 17:33, 5 December 2010 (UTC)
- ..., "oxoazinic acid fully complies with substitutive IUPAC nomenclature as per the red book", ... .
I've finally read the Iranian paper. There is one mention of this: "Oxoazinic acid, urea" in a table. Strangely... why not simply urea nitrate? In a previous entry in the table, they have no difficulty saying "nitrate". PlasmicPhysics: You hang everything on one passing mention by an in-press article? --Rifleman 82 (talk) 17:54, 5 December 2010 (UTC)
- The two uses we have, then, are for HNO3 in combination with other things (ligand, complex, etc.), not "just HNO3" (either neat or in simple solution). I would say that even if we could verify the given examples as proper, they still do not pass muster as good evidence for the name for nitric acid itself or as primary given how rare this use is vs others. Seems just like "ammonia" is NH3 whereas "ammine" is when it's a ligand, and "hydrogen chloride" is HCl but it is often named as a "hydrochloride" component of an amine salt, etc. Again, per Rifleman, editor is making all attempts to rationalize one chosen position in the face of flimsy support for it and strong dissent or evidence for alternatives. DMacks (talk) 18:19, 5 December 2010 (UTC)
- I originally tried provide justification of the legitemicy of oxoazinic acid being the PISN (not PIN), but I revised it and consequently shifted more towards an auxiliary ISN. It may not be the PISN, but it is a ISN none the less. The original argument was not about whether or not to include oxoazinic acid, that is a new direction, the original argument was about whether or not oxoazinic acid was an ISN. We must keep sight of that, so that we may keep this discussion organised. It is hard to provide a citation for all systematic names as there is no singular source, sometimes there simply is no source.
- What I would like to come out of this, is the idea that due to the difficulty of citing PISNs, a dissagreement over a particular PISN must be sorted out on the talk page of that particular article before compusilve reversions occur. Editors should also understand the difference between a PIN and a PISN. Plasmic Physics (talk) 22:30, 5 December 2010 (UTC)
- Oxoazinic acid is an absurd name. In any case not a substitutive name and not a good functional name. An oxoazane would be NH3 with a double bonded O? Moreover, a single cite, even several sources is not enough, so delete the name.--Wickey-nl (talk) 16:49, 6 December 2010 (UTC) And by the way, should Salpetre acid and Spirit of nitre retained?--Wickey-nl (talk) 17:34, 6 December 2010 (UTC)
- No, you don't fully grasp sustitutive nomenclature yet - oxoazane would be an incorrect substitutive name for nitroxyl, the wrong parent group is chosen in this case, the correct substitutive name is oxidanimine. Plasmic Physics (talk) 06:44, 7 December 2010 (UTC)
If there is only websites as source it has to go.--Stone (talk) 07:42, 7 December 2010 (UTC)
- Since oxoazinic acid is not the PISN for nitric acid, I do not contest it's removal, based on the sole fact that it is an relatively unknown other name. If oxoazinic acid was the PISN, I would contest it, since it isn't, I won't. Plasmic Physics (talk) 08:20, 7 December 2010 (UTC)
Molecular diagrams -- is there a WP:CHEMS standard?[edit]
Hi everyone. I noticed that the molecular diagrams often differ quite a bit from article to article. For example, a ball-and-stick diagram is used in Monomethylhydrazine while a quite different "big spheres" model (I'm not a chemist; don't the name for this type) is used for Dinitrogen tetroxide.
Question. Is there any sort of "manual of style" for chemistry molecules on Wikipedia? Is one preferred over the other? Some types deprecated? Different standards for organic and inorganic molecules? Or is it just pretty much article-by-article whatever the editors happened to put in? Thanks for any guidance you can provide. Cheers. N2e (talk) 14:46, 7 December 2010 (UTC)
- There is WP:MOSCHEM, which includes a standard for the skeletal structure diagrams. But the two diagrams you mention actually are different things, not different styles of the same idea. One uses the Van der Waals radius of the atoms, which illustrates the overall shape of the molecule. The other is a ball-and-stick model, in which the atoms themselves are fairly small compared to the bond-lengths to make it easy to see the patterns of connectivity. The diagram in the Van der Waals surface article is a nice comparison of these two. To answer the followup question you haven't yet asked, there is no MOS standard that I know of for either of these two types of diagrams. DMacks (talk) 15:19, 7 December 2010 (UTC)
- Thanks DMacks! That totally answers my question, and gives me a link or two to learn more to boot. Very cool. I don't really have a horse in the race as to whether their ought to be such a standard for Wikipedia molecular articles, or if so, which diagram might make better sense to the reletively non-technical Wikipedia reader. Best wishes. N2e (talk) 20:15, 7 December 2010 (UTC)
- Space-filling models are rather useless elements in a chembox. Mostly they give hardly information about the structure of the molecule and only one perspective of the form.--Wickey-nl (talk) 08:28, 8 December 2010 (UTC)
- I would tend to agree with you Wickey-nl, but then I only took Chems as part of my engineering degree where I focused on Physics, Electronics, and Electrical engineering. The ball and stick models do seem a bit more useful to me as a Wikipedia reader than the large, pregnant Van der Waals radius Space-filling models. That being said, if I read the first commenter correctly, the chemical-interested editors have not developed a consensus on preference for one over the other in the little chem-infoboxes at the top of so many articles. So I figure I must just accept that and move on. However, if a discussion ever starts to revisit the topic, I hope someone will invite me to the discussion. Cheers. N2e (talk) 14:41, 10 December 2010 (UTC)
- I don't know if there has been a discussion at all. I think there is not the question which one is preferred, but if the space-filling model has additional value along with the ball and sticks. The first are nice pictures, that is not the question, but they give very little information. At least front and side view are needed, but for me it isn't worth. On the contrary, for the common reader are ball and sticks less abstract than plain structures.--Wickey-nl (talk) 15:23, 10 December 2010 (UTC)
Plasmic Physics edits[edit]
The damages of User:Plasmic Physics are huge and numerous. In Sulfenic acid he added a Chembox, even with a molar mass for this class of compounds. He also adds systematically PINs, although these are not generally accepted and validated. Please a block.--Wickey-nl (talk) 13:30, 9 December 2010 (UTC)
- I agree that Plasmic Physics contributes vanishingly little to counterbalance his many hurtful edits. But previous complaints from me and others (check out above and his talk page) have only brought conciliation and hand-wringing from our fellow editors, so I gave up complaining and just avoid the issue. Usually an administrator will admonish him/her, there will be a quiet period, and then the activity will resume. --Smokefoot (talk) 13:42, 9 December 2010 (UTC)
Per WP:DR, the next step that should be taken (or actually should have been taken long ago) is to start a user-conduct RFC. The purpose of such an RFC is to a) formally establish that a behaviour problem exists (assuming that one does), and b) bring in third-party views so that this can be better evaluated (RFCs that are properly certified end up listed on a few noticeboards). The general rules for other forms of dispute resolution apply (back up all statements with diffs, make statements about behaviour rather than making ad-hominem attacks, etc.
The results of the RFC (positive and negative) can be referred to as support for any further steps in dispute resolution without having to go through as lengthy an evidence phase, and having performed an RFC (as well as made conversation-based attempts at resolving disagreements) is usually seen as a prerequisite for any more severe stages of WP:DR. --Christopher Thomas (talk) 21:02, 9 December 2010 (UTC)
- Wickey-nl: you're refering to two separate edits, the one in which I added the chembox, is dated several months ago. In the most recent one, all I did was update the chembox that was already there. I added the chembox because, User:Benjah-bmm27 did not make it clear that the article is only about the chemical class and not the compound itself. Chemical articles regularly include both the class and the compound as in Silanol. --Plasmic Physics (talk) 04:37, 10 December 2010 (UTC)
- If you even don't know what a general formula is, please stop editing chemical articles. I don't want you to edit any chembox more, with your stupid standard image widths of 121px [13]. What means ''ImageFileL1_Ref = {{chemboximage|$1|??}}''? I am afraid, no one has verified the many properties additions you made.--Wickey-nl (talk) 10:17, 10 December 2010 (UTC)
- Stop insulting me, I know what a general formula is, it implies nothing. If you can manage to keep up with chembox template modifications, you'll know what ''ImageFileL1_Ref = {{chemboximage|$1|??}}'' is. If the image size bothers you, then fix it, don't bother me about it. --Plasmic Physics (talk) 11:21, 10 December 2010 (UTC)
My contribution to sulfenic acid was creating the article. It is clear from the text that sulfenic acids are a class or family of molecules. If I were referring to a single compound, you'd notice the following differences:
- The article would begin "Sulfenic acid is..." rather than "A sulfenic acid is..."
- "the general formula RSOH" would be replaced with "the formula HSOH" (or whatever the formula of "sulfenic acid" would be)
- "Sulfenic acids are generally unstable" would become "Sulfenic acid is generally unstable"
- "An example of a sulfenic acid is benzenesulfenic acid, PhSOH" would not make sense, so would not be there.
Plasmic Physics, blaming your addition of a chembox on me is not really fair.
Ben (talk) 11:53, 10 December 2010 (UTC)
- It was not a single case, as you can see in this dicussion--Wickey-nl (talk) 15:49, 10 December 2010 (UTC)
- Even the chembox update Plasmic Physics made (given that chembox already existed):
| StdInChI = 1S/HOS/c1-2/h1H
| StdInChI = {{stdinchicite|correct|chemspider}}
| StdInChIKey = ZXYFGZNMDRNOGQ-UHFFFAOYSA-N
| StdInChIKey = {{stdinchicite|correct|chemspider}}}}
- was incorrect. The problem is systematic: so many edits you make are flawed and/or disruptive in so many ways, and you do not recognize it and try to deflect blame. User talk:Plasmic Physics#"Fluoxetine" is an IUPAC preferred name? is a great example. A problem is explained to you, don't read your source carefully so you do not realize the mistake you make. It is explained clearly, you say "anyone can make that mistake." Yes, but the subject is that it is repeatedly you doing it, rising to the level of disruption rather than incremental gain. The net result is that many others spend more time looking over your shoulder than they would actually writing content (WP:AGF, but evidence is that the result is not proper even if the intent is valid). The excuse is "well someone needs to be adding stuff, at least I'm doing it." No, if the "stuff" is so often flawed--quality cannot make up for quality. The amount of hand-wringing is high, the amount of improvement even after reading the comments is low. User does not seem substantially interested in building consensus and working with others, but only in vindication of his own bold ideas (even persisting while non-WP:SNOW discussions of them are on-going!)--see categorization discussion above. DMacks (talk) 16:09, 10 December 2010 (UTC)
- Shoy: I'm annoyed with Wickey-nl for being so desperate to find my supposed mistakes, and stooping so low as to throw insults around.
- Ben: Noted, will be useful for future reference when deciding whether an article is about the chemical compound or class.
- Everyone: The glucose discussion is incomplete in that it is a fraction of the actual discussion on my talk page.
- DMacks: What is wrong with the chembox entries? How sure are you about saying that the edits I make are more diruptive than constructive, compared to my watchlist number of at least 600, what fraction is disruptive? As for the categorization issue, I stoped when asked to. --Plasmic Physics (talk) 20:47, 10 December 2010 (UTC)
No more examples are needed. If this is not enough for a block, we must conclude WP has not enough self-cleaning capacity and is suffering from its own system. Clearly, this user is autistic and without self-reflection (note, this is an explanation, not an attack!).--Wickey-nl (talk) 10:06, 11 December 2010 (UTC)
- Wickey-nl , your comment is insulting. Please exclude yourself from this discussion V8rik (talk) 18:13, 11 December 2010 (UTC)
You are missing the point. Make yourself informed.--Wickey-nl (talk) 17:10, 12 December 2010 (UTC)
- The main job, on top of improving content, is minimizing future damage and undoing the damage done already. On that topic, help is needed to unwind PlasmaPhysics's subcategories within category:organosilicon compounds, wacky things like category:(Trimethylmonosilyl)azanides and category:Monomethylmonosilanes. We can then repopulate category:organosilicon compounds and make the selection of probably 20-40 compounds useful to normal readers.--Smokefoot (talk) 16:59, 11 December 2010 (UTC)
4-[(4-dimethylaminophenyl)-phenyl-methyl]-N,N-dimethyl-aniline[edit]
There are three articles dealing with that chemical
- Crystal violet gives the synthesis but is a stub
- Methyl violet is the article about several similar compounds but the reference to the main article is not clear.
- Gentian violet is the main article, but is mainly focused on the medical applications. At the end it is a manual how to dye skin and jackets.
I will try to help later. All three have room for some improvement.--Stone (talk) 11:11, 24 May 2010 (UTC)
I was thinking of organizing these triarylmethane dyes together. Triarylmethane_dye is a draft. Ullmann's has a nice article on them. This subject seems inherently messy because some of these named substances crude mixtures containing different compounds. That there are so many possible variations seems to make it difficult to cover the topic exhaustively. Would you all think the draft is a good idea? --Rifleman 82 (talk) 04:12, 12 December 2010 (UTC)
- Great idea to edit in this theme because these dyes are used in the real world and their nomenclature and structures are confusing. One thing that I learned is that there are many permutations of C(C6H4NH2-xMex)3 plus substituted derivatives. BTW, if you ever find E1/2s for these things, let me know. --Smokefoot (talk) 23:55, 12 December 2010 (UTC)
Uncategorized chemicals[edit]
There are currently 45 uncategorized chemicals (i.e. articles containing a chembox or a drugbox). I would be grateful to get some help in categorizing them. --Leyo 23:42, 12 December 2010 (UTC)
Any thoughts about the name of this compound versus the structure? I'm thinking of a salt, like the zwitterionic form of amino acids. If it were not a salt, this should not be a single compound. --Rifleman 82 (talk) 08:31, 13 December 2010 (UTC)
- Though the diagram shows two separate compounds - monoethanolamine and oleic acid - there will certainly be a Lowry-Bronsted acid-base reaction between them to form a salt, [CH3(CH2)7CH=CH(CH2)7CO2][H3NCH2CH2OH]. I would name the salt as:
- 2-hydroxyethylammonium oleate or
- 2-hydroxyethylammonium (9Z)-octadecenoate
- Names like "monoethanolamine oleate" are examples of careless nomenclature in the pharmaceutical industry. It implies that the oleate is present as a salt or an ester, but the monoethanolamine implies a stand-alone neutral compound. EdChem (talk) 09:51, 13 December 2010 (UTC)
Thanks for that. I'll go fix it. --Rifleman 82 (talk) 09:59, 13 December 2010 (UTC)
Thanks to all for the help. There are only 3 articles left: Multiferon, Tidiacic arginine, Proligestone. --Leyo 13:03, 14 December 2010 (UTC)
Iron Hydride and Chromium Hydride[edit]
I could not find articles on this. Is there any information on this, cause been trying to find info. Example page which talks about the chemical but does not explain the chemical is, Wolf_359#Outer_atmosphere. Thanks, Marasama (talk) 18:07, 14 December 2010 (UTC)
- The binary compounds of hydrogen article will explain iron hydride does not exist. Like you I am interested in info on chromium hydride V8rik (talk) 18:13, 14 December 2010 (UTC)
- There is some literature on iron hydride, all related to geochemistry/geophysics at extreme conditions. DMacks (talk) 18:17, 14 December 2010 (UTC)
- I have added iron hydride, there is actually quite a bit of literature. Graeme Bartlett (talk) 13:18, 22 April 2012 (UTC)
- And now chromium hydride Graeme Bartlett (talk) 09:50, 5 August 2012 (UTC)
- I have added iron hydride, there is actually quite a bit of literature. Graeme Bartlett (talk) 13:18, 22 April 2012 (UTC)
- There is some literature on iron hydride, all related to geochemistry/geophysics at extreme conditions. DMacks (talk) 18:17, 14 December 2010 (UTC)
Chembox[edit]
How does the field, IUPACName, differ from both PIN and SystematicName fields? --Plasmic Physics (talk) 04:48, 10 December 2010 (UTC)
- If no one recognises this field, then I propose to phase this field out and eventually delete it. -Plasmic Physics (talk) 07:35, 13 December 2010 (UTC)
Ask Beetstra. If I recall, this is for the IUPAC name. Then we had some people trying to put "ethanoic acid" in there where it should be acetic acid. So we had systematic name, which uses the rules but does not recognize the retained names. Then PINs came along. --Rifleman 82 (talk) 08:23, 13 December 2010 (UTC)
Sigh. All three can be different, but PIN should finally be one that is 'correct'/'systematic' - for now, IUPACName (acetone) and SystematicName (2-propanone), should be fine. There is no reason to phase out anything, when we have all filled and verified, and then do a check to see whether SystematicName is still useful, then we do that. Indeed, PINs are not yet officially implemented (though some parts of the draft will not change too much anymore, so those could be used). Wickey-nl - I added it just to be ahead of those things, there is no problem in having parameters already there while they are not used yet, Plasmic Physics, PIN is there as it is a clean sheet, which would make it easier to fill them from scratch in terms of verification. We might actually get to the point where most of them will be set in a scripted way, from verified StdInChI's, e.g. --Dirk Beetstra T C 17:41, 13 December 2010 (UTC) (wrong date, forgot to sign)
- Correct, but only when the term systematic name is used in broader sense. Oxytocin is a PIN, not (2S)-1-[(4R,7S,10S,13S,16S,19R)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-[(2S)-butan-2-yl]-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-N-[(2S)-1-[(2-amino-2-oxoethyl)amino]-4-methyl-1-oxopentan-2-yl]pyrrolidine-2-carboxamide.
- Verifying is rather awkward--Wickey-nl (talk) 16:08, 13 December 2010 (UTC)
- The PIN should be a one-on-one match, so it has a 'correct' form. That type of info can be 'verified', and followed/checked by CheMoBot. Many others are more 'flavoured' (different correct IUPACNames can exist for a compounds). I think your example could be one, if the drug has a INN, which we would use as article title, and that would be an IUPACName, the systematic name would be the full worked-out name, and the PIN might differ from the INN. Voila, all three filled. --Dirk Beetstra T C 17:41, 13 December 2010 (UTC)
- The PIN field can remain, but I stay in my opinion that using it yet is premature, because they still are not recommended. Moreover, if someone gets the idea (you never know) to change all IUPAC names into PINs, there is no simple way to verify them. I will not do that job.--Wickey-nl (talk) 11:57, 14 December 2010 (UTC)
- As I said, we can do that scripted, e.g. on the basis of StdInChI's (similar to what I am doing now) .. I'll just overwrite anything else at that point. That being said, yeah, if you're not sure if it is going to stay the prefered IUPAC name, then filling it in is futile, and not too smart. --Dirk Beetstra T C 19:01, 14 December 2010 (UTC)
- You can expand InChI's to images, and images can be rendered into names. It will not be totally fool proof, but the name-generating-software is there (now only waiting for PINs to become properly available). --Dirk Beetstra T C 12:08, 15 December 2010 (UTC)
- Sorry, I should have said 'You can expand InChI's to images, and images can be rendered into PINs'. --Dirk Beetstra T C 12:19, 15 December 2010 (UTC)
- Or in whatever way, fact remains, the PIN is uniquely correct for a certain compound, and that is what CheMoBot can keep an eye on in the end. --Dirk Beetstra T C 12:20, 15 December 2010 (UTC)
Zinc pyrithione structure confusion[edit]
I just re-discovered the long-standing confusion about what the structure of Zinc pyrithione is--how many pyrithione ligands are on the zinc center? Definitely several sane possibilities, but the article and infobox appear to be merging them as if they were a single chemical, and the linked pubchem and chemspider pages only perpetuate the mixup. Could someone take a peek and try to help sort it out? DMacks (talk) 17:15, 15 December 2010 (UTC)
- The ChemSpider ID was obviously wrong: I have corrected it. As for the rest, the article seems quite clear to me: the compound is a dimer in the solid state but a monomer in solution. That in itself is not particularly unusual behaviour, so people should speak up if they have any strong feelings as to how we should handle it. Physchim62 (talk) 18:20, 15 December 2010 (UTC)
- It's not just [pyr2Zn] vs [pyr2Zn]2 (organometallics are weird polymeric forms, don't really need to deal with that larger issue) but more seriously [pyr2Zn] vs [pyr1Zn] (forgive my shorthand for the structures here). For example, CID 10313492 and SMILES [Zn+2].S=C1/C=C\C=C/N1O. Should be CID 26041 or CID 3005837 but I don't understand the difference (or reliability/uniqueness/etc of pubchem id in general). DMacks (talk) 18:51, 15 December 2010 (UTC)
- I see no chemical reason for [pyr1Zn] to exist: it appears to be a figment of PubChem's imagination (it wouldn't be the first time). Physchim62 (talk) 19:12, 15 December 2010 (UTC)
- It's not just [pyr2Zn] vs [pyr2Zn]2 (organometallics are weird polymeric forms, don't really need to deal with that larger issue) but more seriously [pyr2Zn] vs [pyr1Zn] (forgive my shorthand for the structures here). For example, CID 10313492 and SMILES [Zn+2].S=C1/C=C\C=C/N1O. Should be CID 26041 or CID 3005837 but I don't understand the difference (or reliability/uniqueness/etc of pubchem id in general). DMacks (talk) 18:51, 15 December 2010 (UTC)
Technically a coordination compound, not an OM compound... --Rifleman 82 (talk) 19:16, 15 December 2010 (UTC)
Block for Plasmic Physics[edit]
I have requested a block for User talk:Plasmic Physics: Wikipedia:Administrator_intervention_against_vandalism--Wickey-nl (talk) 14:08, 21 December 2010 (UTC)
Plasmic Physics is blocked for a week. Please take action if he restarts disruptive editing.--Wickey-nl (talk) 11:17, 22 December 2010 (UTC)
Pages to review: Imidogen; Tris(tert-butoxy)silanethiol; Dimethyldichlorosilane; Disiloxane (data page);Fluoromethylidyne; Dihydroxymethylidene; Disiloxane; Template:Infobox untriseptium.--Wickey-nl (talk) 14:32, 22 December 2010 (UTC)
More clean-up to do:[edit]
Other works already in progress or to follow for even more common substances:
- Methanidylidyneoxidanium: systematic name invented for carbon monoxide has been suppressed by Dirac66;
- Methanedione used as systematic name for carbon dioxide was also suppressed but is presently under discussion at Talk:Carbon_dioxide#Methanedione_and_Dioxidocarbon;
- Beryllium oxide was also affected here with
| SystematicName = Oxidaniumylidyneberylliumuide
- recently modified in:
| SystematicName = Beryllio-λ1-oxidanyl (substitutive)
Still many clean-up remaining to do. When necessary, short but appropriate and convincing arguments should be left on the respective discussion pages to avoid edit wars. It seems he finally accept to discuss (a positive point), but care should be taken not to engage in sterile and useless dialectics. It must be done efficiently and correctly with a fair attitude to leave no grip to endless discussion. The support of other editors (or administrators) is certainly needed. Remain neutral and patient. Good work and Best regards, Shinkolobwe (talk) 23:35, 22 December 2010 (UTC)
Problematic category: Oxosilicon[edit]
Category:Oxosilicons created by User:Plasmic Physics is making a double use with the existing Category:Silicon dioxide. I propose to delete Category:Oxosilicons which does not make sense. Shinkolobwe (talk) 22:18, 25 December 2010 (UTC)
- Agreed, but also a suggestion - why not rename the Category:Silicon dioxide to Silicon oxides - then it can encompass Silicon monoxide. Ronhjones (Talk) 00:27, 26 December 2010 (UTC)
Gold Book citations[edit]
The Gold Book is often cited in chemical articles. There is some dispute about the Template:GoldBookRef. Should it be cited by the unambiguous short name that IUPAC uses (the "Gold Book"), or should we overload the reference sections with the unknown full name "IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006-)"?--Wickey-nl (talk) 11:37, 22 December 2010 (UTC)
- We write in a formal style, and citations should correspondingly not be informal. --Rifleman 82 (talk) 08:54, 23 December 2010 (UTC)
- A short cite which gives all needed details with one simple click is appropiate. But off course "IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006-)" gives the nice feeling and illusion you are working on a scientific article in a scientific encyclopedia.--Wickey-nl (talk) 09:32, 24 December 2010 (UTC)
- When hundreds of (chemistry related) articles have no proper refs at all, I won't waste time on arguing about the formatting of one. Materialscientist (talk) 09:41, 24 December 2010 (UTC)
- A short cite which gives all needed details with one simple click is appropiate. But off course "IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006-)" gives the nice feeling and illusion you are working on a scientific article in a scientific encyclopedia.--Wickey-nl (talk) 09:32, 24 December 2010 (UTC)
Problematic use of SystematicName for Beryllium oxide[edit]
See hereafter, an excerpt from the chembox of beryllium oxide:
| ImageName = Unit cell ball and stick model of beryllium oxide
| IUPACName = Beryllium oxide
| SystematicName = Beryllio-λ1-oxidanyl (substitutive)
Oxidoberyllium (additive)
Question: Is it meaningful to present the substitutive name as systematic name for beryllium oxide in Wikipedia ? The composition name and the additive systematic names are both simple and very clear. However, the substitutive name does not seem to make sense to describe a solid compound made of a hexagonal network of beryllium cations and oxide anions. Such a complex name would be appropriate to describe the structure of a single molecule (i.e., an isolated moiety), should this one exist, but not for describing the unit cell repeated in a crystalline network. It seems that here the nomenclature logic is once again pushed behind its reasonable limits by User:Plasmic Physics. I have not verified myself with the IUPAC Redbook (IUPAC nomenclature of inorganic chemistry 2005) if the substitutive naming is correct or not.
The immediate consequence of systematically extending the use of such useless names (if not incorrect at all) to all inorganic compounds in Wikipedia is to puzzle even more the reader than to help him. Nearly nobody will use this intricate nomenclature and the risk to mislead the audience is not negligible.
It is worth to question us before accepting to altering/polluting thousands of chemboxes with such low-value information. Again here, common sense and self-critic mind are necessary: what is the the aim and the added value of such overspecialized edits. My opinion is that when writing often less is more. Thank you for your opinions and comments. Best regards, Shinkolobwe (talk) 17:37, 25 December 2010 (UTC)
- I occasionally undo or remove Plasmaphysic's edits when I am convinced the work does not serve readers. Plasma is into esoteric and/or ultra-formal nomenclature and categorization resulting in contributions that are perplexing to experienced and novice readers alike. Like you say, more is less in some cases. Oh well...--Smokefoot (talk) 02:48, 26 December 2010 (UTC)
Substitutive IUPAC names are most often not appropriate for inorganic compounds[edit]
Hereafter, a first excerpt from the IUPAC Redbook (see p. 5 on paper, p. 17 in the PDF file):
IR-1.5.3 Systems of nomenclature | IR-1.5.3.1 General: Three systems are of primary importance in inorganic chemistry, namely compositional, substitutive and additive nomenclature; they are described in more detail in Chapters IR-5, IR-6 and IR-7, respectively. Additive nomenclature is perhaps the most generally applicable in inorganic chemistry, but substitutive nomenclature may be applied in appropriate areas. These two systems require knowledge of the constitution (connectivity) of the compound or species being named. If only the stoichiometry or composition of a compound is known or to be communicated, compositional nomenclature is used.
Most of the problem recently encountered for the nomenclature of simple inorganic compounds such as different silicate obviously arose from a wrong interpretation of the IUPAC nomenclature system by User:Plasmic Physics. For inorganic compounds the compositional nomenclature (the simplest and the more classic one) is most often preferable and also officially recommended by IUPAC. The additive nomenclature which is more complex comes in second rank. Finally, the substitutive nomenclature is the less general nomenclature for inorganic compounds and previously requires the knowledge of the connectivity of the compound or species being named.
Hereafter, a second excerpt from the IUPAC Redbook (see p. 6 on paper, p. 18 in the PDF file):
IR-1.5.3.3 Substitutive nomenclature: Substitutive nomenclature is used extensively for organic compounds, … . In particular it is used for naming organic ligands in the nomenclature of coordination and organometallic compounds, …
So, the substitutive nomenclature is only useful when an inorganic ligand is part of a more complex bio-organic or organo-metallic entity. This kind of complex nomenclature is best avoided if not necessary even in scientific publications. Most often, it is reserved to the only publications describing the first synthesis or the first determination of the crystallographic structure of a new compound. Interestingly, IUPAC itself acknowledges the fact that its own conventions may differ from these of the International Union of Crystallography and that the guidelines given in the Redbook are not definitive. This emphasizes the relativity of nomenclature rules.
This explains why most of the contributors to the Project Chemicals are strongly perturbed by the recent edits made by User:Plasmic Physics. Practically, I would propose to ban the use of substitutive IUPAC nomenclature for inorganic compounds when it is clearly not applicable or simply not useful. The compositional IUPAC nomenclature should be first privileged and if considered useful, the additive IUPAC nomenclature rule might be given. This solution could relief the recent tensions caused by the blind application of non-appropriate IUPAC rules in the chemboxes of inorganic compounds by User:Plasmic Physics.
Naturally, for organic substances, the substitutive IUPAC nomenclature certainly makes sense, and no limitations should be applied in the field of organic chemistry. Please, simply read the introduction of the Redbook which explains how to correctly use it. The most shocking feeling with the very complex substitutive IUPAC nomenclature rules applied to very simple inorganic compounds is that they all appear to be treated without reason as complex carbon-based organic molecules. And this non-necessary overcomplexity is highly disruptive for very simple inorganic molecules. Best regards, Shinkolobwe (talk) 17:16, 28 December 2010 (UTC)
Example of inappropriate application of the substitutive IUPAC nomenclature for sodium silicate: see the chembox of sodium silicate and the discussion at the Talk page of sodium silicate. Disodium oxosilanebis(olate) (substitutive nomenclature) is simply not applicable for simple inorganic silicon compounds. Shinkolobwe (talk) 17:24, 28 December 2010 (UTC)
OR introduction of systematic names[edit]
Wikipedia:No original research is giving everything you need to know. I will delete all systematic names I can find which are not sourced. IUPAC red yellow green gold or what ever colour book does not qualify, because it is a rule book to create the name not a source. There is not one reliable source using the name
- Dioxoprotactinium
- Dioxidoprotactinium
- Beryllio-λ1-oxidanyl
- Disodium oxosilanebis(olate)
This use of unsourced material is OR there no way that OR gets a place in any science articles. --Stone (talk) 17:43, 28 December 2010 (UTC)
- Sodium amide: Sodium dihydridonitrate(1-) (additive) (this) and Sodium μ-nitrido-dihydrogenate(1-) (additive) (this) make also absolutely no sense. Shinkolobwe (talk) 18:33, 28 December 2010 (UTC)
- Nitric acid: Hydroxidodioxidonitrogen
- Magnesium trisilicate: Dimagnesium {[dioxido({[oxido(oxo)silyl]oxy})silyl]oxy}silanoylolate
--Stone (talk) 19:17, 28 December 2010 (UTC)
- Silicon monoxide: oxidanylsilanylidyne
--Shinkolobwe (talk) 23:45, 28 December 2010 (UTC)
- Oh dear. Plasmic Physics, please stop it, you know wikipedia and chemistry better than many other users. So many chemistry articles really need your help with references, addition of basic properties, or just simply vandalism reversion. Materialscientist (talk) 00:11, 29 December 2010 (UTC)
Cleanup of overspecialized silanes categories created by User:Plasmic Physics[edit]
I have tried to remove most of the overspecialized sub-silanes categories recently created by User:Plasmic Physics. I replaced most of them simply by Category:Silanes which is presently not too populated. It seems reasonable and now all silanes are easily seen together and the discovery of other similar molecules is much easier than with the exaggerated arborescence made by User:Plasmic Physics. I think the job should be almost done, but a quick control by other contributors would be appreciated. These empty categories are now labelled with the category Empty category waiting for deletion. I hope that some administrators will see it soon and delete them without too much waiting. Shinkolobwe (talk) 12:05, 30 December 2010 (UTC)
- Empty categories should not be placed in the category Category:Empty categories awaiting deletion, but tagged with the speedy deletion tag {{db-emptycat}}. Thanks. --Starcheerspeaksnewslostwars (talk) 18:20, 30 December 2010 (UTC)
- Thank you, for the information. I am sorry, I was not aware of the difference. Shinkolobwe (talk) 18:25, 30 December 2010 (UTC)
- No problem. Glad to help out and I know that's all you were doing. Not sure what the guy who created them all was thinking. Were they ever populated? Looks like some just go from one sub-category to another. --Starcheerspeaksnewslostwars (talk) 18:28, 30 December 2010 (UTC)
 Done You tag 'em, and I'll delete 'em Ronhjones (Talk) 19:30, 30 December 2010 (UTC)
Done You tag 'em, and I'll delete 'em Ronhjones (Talk) 19:30, 30 December 2010 (UTC)
- Thank you. Still one sub-silane category awaiting deletion: Category:Monohalomonosilanes. Shinkolobwe (talk) 21:10, 30 December 2010 (UTC)
- Thank you, for the information. I am sorry, I was not aware of the difference. Shinkolobwe (talk) 18:25, 30 December 2010 (UTC)
- ^ Keshavarz, Mohammad Hossein (2010). "A new approach to predict the strength of high energy materials". Journal of Hazardous Materials. In Press, Corrected Proof. doi:10.1016/j.jhazmat.2010.10.093. ISSN 0304-3894. Retrieved 2010-12-04.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)