Wikipedia:Reference desk/Archives/Science/2012 June 21
| Science desk | ||
|---|---|---|
| < June 20 | << May | June | Jul >> | June 22 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
June 21[edit]
Boiling hotter, boiling faster?[edit]
It's a commonly noted fact that salted water boils hotter than unsalted water. But my question is, does it boil faster? If I have a pot of salted water, will it boil away faster than an otherwise identical pot of unsalted water? If I synchronize the pots by when I turn the flame on? If I synchronize the pots by when they begin to boil? Thanks. Someguy1221 (talk) 00:38, 21 June 2012 (UTC)
- Possibly of interest. Short Brigade Harvester Boris (talk) 00:45, 21 June 2012 (UTC)
- Assuming both start at ambient temperature, the salted water will boil slightly slower, since it has to heat up further to reach it's boiling point. 203.27.72.5 (talk) 00:47, 21 June 2012 (UTC)
I suppose another consideration is that salt water has more entropy than non-salted, so it will resist evaporation. That will lead to it losing less heat and possibly boiling faster. That will of course depend on the concentration of salt and ambient humidity, etc.203.27.72.5 (talk) 00:50, 21 June 2012 (UTC)- Scratch that. The higher entropy of the salt water is what makes the boiling point higher to start with. The amount evaporating will still increase as the temperature approaches the boiling point. The salt water will boil ever so slightly slower, and hotter. As an aside, any food in the pot will cook faster in boiling water at 100°C than it will in not-yet-boiling-salt-water at 100°C. This is because steam at 100°C has much more internal energy than water and can transfer it to the food faster through the agitation of a rolling boil. 203.27.72.5 (talk) 01:08, 21 June 2012 (UTC)
- Keep in mind that a given volume of pure water actually contains more H2O than the same volume of salt water. Because salt water is an imperfect mixture, NaCl (or rather, its dissolved ions) represents a statistically significant portion of the "water", and salt does not boil at 100C. The specific thermic properties of NaCl notwithstanding, you're actually starting off with less water to begin with in the case of salt water. I'll let people smarter than me tell you what that means in practical terms. Evanh2008 (talk|contribs) 01:16, 21 June 2012 (UTC)
- In practical terms, nothing. Dissolving NaCl in water doesn't appreciably change its volume, which, incidentally, is why brine is much denser than water and tends to sink. This drives various climatic processes as I understand it. 203.27.72.5 (talk) 01:49, 21 June 2012 (UTC)
- Keep in mind that a given volume of pure water actually contains more H2O than the same volume of salt water. Because salt water is an imperfect mixture, NaCl (or rather, its dissolved ions) represents a statistically significant portion of the "water", and salt does not boil at 100C. The specific thermic properties of NaCl notwithstanding, you're actually starting off with less water to begin with in the case of salt water. I'll let people smarter than me tell you what that means in practical terms. Evanh2008 (talk|contribs) 01:16, 21 June 2012 (UTC)
- Scratch that. The higher entropy of the salt water is what makes the boiling point higher to start with. The amount evaporating will still increase as the temperature approaches the boiling point. The salt water will boil ever so slightly slower, and hotter. As an aside, any food in the pot will cook faster in boiling water at 100°C than it will in not-yet-boiling-salt-water at 100°C. This is because steam at 100°C has much more internal energy than water and can transfer it to the food faster through the agitation of a rolling boil. 203.27.72.5 (talk) 01:08, 21 June 2012 (UTC)
Using genetics to determine the potential for undiscovered species[edit]
I often read stories about a new species of plant/animal being discovered. My question is: Can the genetic code of a plant/animal specimen provide clues to the existence of a yet-to-be discovered species. Take tree frogs, for example. They cover a pretty wide range of variations and species, and it is likely a new species is yet to be discovered. Could a scientist interested in tree frogs look at the genes of a known species, and extrapolate the potential for other, undiscovered species of tree frogs to exist...or at least the potential to exist? Quinn ✹SUNSHINE 03:23, 21 June 2012 (UTC)
- Well, sure -- the potential, not the actuality. But I suspect if you knew more about genetics you would realize that that fact is not very interesting. People who do genetic engineering invent new species every day -- many of them have the potential to exist before they are created, in a theoretical sense. The number of viable gene combinations is unimaginably huge. Looie496 (talk) 03:45, 21 June 2012 (UTC)
- Do genetic engineers really make new species? As in, they modify the genetic material to such an extent that a population of organisms with the modified genetic material cannot mate with the general population of organisms with natural genetic material to produce viable offspring (but can produce viable offspring within their own population). I was not aware of that being possible. 203.27.72.5 (talk) 04:06, 21 June 2012 (UTC)
- It's possible. Take, for example, a very extreme case of engineered underdominance, described in theoretical capacity in this paper, section 5 if you can access it. You can also cheat and just make an organism incapable of mating for some stupid reason, like engineering a self-fertilizing nematode to have no opening through which to mate. It's self-fertilizing, so it's still viable, but now reproductively isolated. And finally, what is actually done, but may not count as "engineering", us using colchicine treatment to change the copy number of chromosomes in a plant. The resulting plant cannot yield anything but sterile offspring with the plant of origin (as the progeny will have a sterility-inducing odd number of chromosomes). So in that sense, it is at least reproductively isolated, if not a new species. You decide if that's genetic engineering. Someguy1221 (talk) 04:22, 21 June 2012 (UTC)
- Do genetic engineers really make new species? As in, they modify the genetic material to such an extent that a population of organisms with the modified genetic material cannot mate with the general population of organisms with natural genetic material to produce viable offspring (but can produce viable offspring within their own population). I was not aware of that being possible. 203.27.72.5 (talk) 04:06, 21 June 2012 (UTC)
Answering the original question, yes, it can provide clues. You can take any two species, there were almost certainly intermediate genetic steps between those two organisms and their most recent common ancestor. Any one of those intermediate organisms may have itself given rise to more than one descendant species. So when you are looking at a phylogeny and there appears to be a large gap between two closest relatives, a biologist will often suspect that some extra species might fit in there. For example, Caenorhabditis elegans has essentially no close relatives (at least by comparison to other members of Caenorhabditis), and there is currently a $5000 prize offered to whichever lucky field biologist finds a species that is more closely related to elegans than anything else. But there are a couple problems with this. It's possible that all of those intermediate forms separating two closest relatives may have not given rise to any other species, or alternatively, those other species might all be extinct. Someguy1221 (talk) 04:31, 21 June 2012 (UTC)
Solar Energy and Its Production[edit]
How many units are present in 1 KW of electricity? What is the present cost of the project for producing 1 MW of electricity with the help of solar panels, etc. without the inclusion of the cost of land? — Preceding unsigned comment added by 122.172.176.45 (talk) 04:07, 21 June 2012 (UTC)
- 1 kW is 1000 Watts of power. A Watt is 1 Joule per second. A Joule is the energy required to accelerate an object with a mass of 1 kilogram at 1 meter per second per second for 1 second. The kilowatt is therefor a derived unit, and is a combination of the base units kilograms, meters, and seconds. You can also express it in terms of these base units as Mg·m2/s3. 203.27.72.5 (talk) 04:13, 21 June 2012 (UTC)
- As for your second question, it depends on where the solar panels will be located. Different areas have very different incident sunlight. Solar panels will also cost different amounts in different places due to logistics, supply of raw materials, labour, government subsidies, etc. 203.27.72.5 (talk) 04:25, 21 June 2012 (UTC)
- Solar panels vary considerably but a solar panel that can produce 250W measures about 1m x 1.6m, you'd need 4000 of those to get 1MW under ideal conditions. Expect to pay $500 for such a panel and you get about 2 million bucks for the solar panels alone. It would also take up about 6400 square meters of space if they were installed edge to edge, which I'm sure is probably not practical. Then you have to install them and also all the infrastructure needed to collect and condition and invert and whatever else you need to do, I wouldn't be surprised if it was another 2 million. Having said that, I'm sure you could get a good deal if you were buying $4 Million dollars of solar power equipment, but this is only intended as a very rough ballpark figure. Vespine (talk) 05:08, 21 June 2012 (UTC)
- To put that in perspective, a rugby field minus the try zone is 6800m2.
- Solar panels vary considerably but a solar panel that can produce 250W measures about 1m x 1.6m, you'd need 4000 of those to get 1MW under ideal conditions. Expect to pay $500 for such a panel and you get about 2 million bucks for the solar panels alone. It would also take up about 6400 square meters of space if they were installed edge to edge, which I'm sure is probably not practical. Then you have to install them and also all the infrastructure needed to collect and condition and invert and whatever else you need to do, I wouldn't be surprised if it was another 2 million. Having said that, I'm sure you could get a good deal if you were buying $4 Million dollars of solar power equipment, but this is only intended as a very rough ballpark figure. Vespine (talk) 05:08, 21 June 2012 (UTC)
- As for your second question, it depends on where the solar panels will be located. Different areas have very different incident sunlight. Solar panels will also cost different amounts in different places due to logistics, supply of raw materials, labour, government subsidies, etc. 203.27.72.5 (talk) 04:25, 21 June 2012 (UTC)
- By "units" the OP may be refering to the terminology used by power companies/authorities when charging for the use of electricity. For example, in Australia, 1 Unit is 1kw for 1 hour - about the energy consumed in one hour by 17 60 watt incadescent light globes, or the energy consumed in one hour by a small bar radiator. As to the OP's second question, the answer given above is entirely right, however, without govt subsidies, solar power is so costly compared to fossil fuel generation, no same person would ever consider it, unless fossil fuel generation cannot be used at the location. The unsubsidised cost of panels, electronics, batteries, installation, etc, for 1 kw for 24 hours a day is of the order of $200,000, with a service life of about 10 to 15 years. By using solar power to back feed power into the grid, rather than owner-conmsumption, batteries are eliminated, however without govt subsidies and a value per unit (kw-hr) of the order of 6 to 10 times the normal electricity charge, it is not cost effective. Ratbone121.215.67.177 (talk) 05:23, 21 June 2012 (UTC)
- So using that definition of unit, 1kW is 1/3600 units. 203.27.72.5 (talk) 05:27, 21 June 2012 (UTC)
- No. Kilowatts (kw) are the units of power. Units, as used by power companies, are the units of energy. Energy is power multiplied by time. 1 kw for 1 hour (or 2 kw for 1/2 hour, etc) is 1 Unit, as I previously stated. By your use of 1/3600, you possibly were thinking of 1 kw.second. Ratbone121.215.67.177 (talk) 06:44, 21 June 2012 (UTC)
- Yeah, so
- 1kW is the energy per 1 second, and over the hour that sums to 3600kJ. I didn't express myself very clearly though. What I should have said was, "you can't ask for a conversion factor between power and energy. That's like asking for a converstion factor between distance and speed." 203.27.72.5 (talk) 08:20, 21 June 2012 (UTC)
- Yes, and now that we have the diffrence between power and energy sorted out, you can see that Vespine's answer is really nonsense. I could use solar panels of arbitarily small area to charge a storage device (battery or capacitor), and then discharge it thru a very low resitance. The power upon discharge could be a megawatt, if only for a microsecond. Ratbone58.167.254.173 (talk) 16:53, 21 June 2012 (UTC)
- I wouldn't say it's complete nonsense. It calculates the minimum size required to generate 1MW continuously, assuming continuous sunlight. Of course in reality, we don't have continuous sunlight, so to replace a 1MW gas turbine it would need to be far larger than his estimate and it would need to store most of its energy for later consumption (peak energy requirements for most areas are in the early evening). Since the OP is asking for a cost of all of the components excluding land, perhaps his idea is to stick solar panels on a large number of houses? If this is the case you'd also have to account for the slope of the roof and how it affects the amount of sunlight incident on the panel. 203.27.72.5 (talk) 20:44, 21 June 2012 (UTC)
- Evidently, wikipedia has an article on this topic. 203.27.72.5 (talk) 08:23, 21 June 2012 (UTC)
- There is also an article on the Kilowatt hour. Jørgen (talk) 08:44, 21 June 2012 (UTC)
- Yes, and now that we have the diffrence between power and energy sorted out, you can see that Vespine's answer is really nonsense. I could use solar panels of arbitarily small area to charge a storage device (battery or capacitor), and then discharge it thru a very low resitance. The power upon discharge could be a megawatt, if only for a microsecond. Ratbone58.167.254.173 (talk) 16:53, 21 June 2012 (UTC)
- Yeah, so
- No. Kilowatts (kw) are the units of power. Units, as used by power companies, are the units of energy. Energy is power multiplied by time. 1 kw for 1 hour (or 2 kw for 1/2 hour, etc) is 1 Unit, as I previously stated. By your use of 1/3600, you possibly were thinking of 1 kw.second. Ratbone121.215.67.177 (talk) 06:44, 21 June 2012 (UTC)
- So using that definition of unit, 1kW is 1/3600 units. 203.27.72.5 (talk) 05:27, 21 June 2012 (UTC)
What is RNA ‘Ribonucleic acid’ and what is DNA 'Deoxyribonucleic Acid'[edit]
What is RNA ‘Ribonucleic acid’ and what is DNA 'Deoxyribonucleic Acid' — Preceding unsigned comment added by 175.140.179.46 (talk) 06:41, 21 June 2012 (UTC)
- Have you checked the articles DNA or RNA? I imagine they would have something to say on the subject. Evanh2008 (talk|contribs) 06:43, 21 June 2012 (UTC)
Cells~~(*for cells questions)[edit]
What are the three main categories of organelles within the cytoplasm? — Preceding unsigned comment added by 175.140.179.46 (talk) 06:48, 21 June 2012 (UTC)
- 1) Those which do their own homework.
- 2) Those which call other cells on their cell phone to get homework answers.
- 3) Those which try to get people on Wikipedia to do their homework for them. StuRat (talk) 06:57, 21 June 2012 (UTC)
- Looking at organelle, I was disappointed to discover that they aren't female organs, but perhaps that article will have the answers you seek. However, at first glance, I only see two categories listed, but definitions may vary, so I suggest you consult your textbook. StuRat (talk) 07:01, 21 June 2012 (UTC)
- Now you made me look up "female organs"... 92.80.52.54 (talk) 08:26, 21 June 2012 (UTC)
after classifying plants[edit]
In the novel La tournée de Dios by Enrique Jardiel Poncela God comes down to Earth and, well, stuff happens. Anyway, in one of the chapters God is invited to a botanical garden and ask to the staff "and when you have classified all plants and animals, then what?"
Anybody has a good answer to God?--85.55.200.82 (talk) 10:58, 21 June 2012 (UTC)
- Then we start to make our own animals and plants by genetic engineering! But I expect that God would point out that humans have not looked every where yet for life forms, especially at other planets. Graeme Bartlett (talk) 12:06, 21 June 2012 (UTC)
- There's a lot to untangle here. First: taxonomy is a distinctly human concept, and our taxonomies are constantly being rearranged, due to new information and new techniques (e.g. systematics, phylogenetics and cladistics are used much more currently, compared to the morphological comparisons used in the past. Second: life form themselves are constantly speciating, and I'm not sure that the rate of classification of new species is greater than the worldwide rate of speciation. So, it is entirely possible (and IMO likely) that we will never "finish" classifying life. Finally: suppose we did. It is a very narrow view of botany and zoology to think that classification is a primary concern (surely God would know better ;). In the modern era, much more research is done in other areas. Even if we had classified all life (and it stopped speciating), we'd have plenty left to discover. How do birds migrate? How does bark form on trees? Why don't naked mole rats get cancer? How do symbioses form? How does a bee hive function collectively? These are all very simple questions that have not yet been completely answered. Actually I would be more concerned that rigorous classification is getting too little attention these days; I've heard many biologists lament that the last of the great taxonomists are dying, and the younger generation is not filling in the ranks. SemanticMantis (talk) 13:34, 21 June 2012 (UTC)
- Why? Despite many biologists wishing it weren't so? I can understand if this was something like prewar-era jazz singer or newspaper editor or something but why this? Is it funded completely by lint taxes? Is the rest of biology so compelling that no biology major/grad student has ever been able to resist the power of the dark side? They're people who're most interested in computational linguistics or train timetables for goodness sake, why not this? Are there no science types left who're also into 18th/19th century stuff? Doesn't seem like it should be the case as some young people still like Steampunk. And aren't there British biologists? And British people should be more likely to like old stuff than Americans. Sagittarian Milky Way (talk) 17:43, 22 June 2012 (UTC)
- Nice try, Semantic. God is obviously a young earth creationist, so he doesn't believe in speciation. 203.27.72.5 (talk) 20:56, 21 June 2012 (UTC)
- Ha! Well, I'm not here to discuss religious beliefs, but suffice it to say that not all who believe in a god believe in YEC. Unfortunately, it seems to be the case that the craziest religious people make the most noise :-/ SemanticMantis (talk) 21:28, 21 June 2012 (UTC)
"Be patient and get in the queue behind the insects, we'll get round to You when we're ready." DriveByWire (talk) 23:35, 24 June 2012 (UTC)
"God, you know more about evolution than we do. You know the process of speciation is endless and ongoing, and the number of small population species of smaller creatures (insects, bacteria, etc) is astronomical. We will never finish cataloging life, your grandest creation, as you well know. However, we can set a new goal of sequencing the DNA of every species, along with cataloging all the proteins that exist. Thank you for the Periodic Table and our ability to create computers based on the electrical and chemical properties of Silicon, with which we can decode the molecules of life." in the Hebrew tradition, god welcomes challenges from us to his questions.(mercurywoodrose)50.193.19.66 (talk) 17:02, 25 June 2012 (UTC)
TV with a particular type of EPG[edit]
I am in the UK. I need to buy a small television, and I have only one requirement and that is that the current live TV channel is displayed within a small window in the EPG, i.e. I can continue to watch and listen while browsing the channels. Unfortunately this information is missing from almost all the online descriptions of the many different models available, and the manufacturers' websites turn out to be useless. The only comparison chart I've been able to find is this one for video recorders, and it only lists a few obscure brands and none of the big names. However, I strongly suspect that is the manufacturer that is significant rather than the TV model, as they tend to use basically the same software for much of their range. My trusty Philips set-top box does what I want perfectly. But I have looked at friends' Sanyo, Toshiba, and JVC TVs, and they are no good, so it seems that what I want is a minority feature. Before you say ask the salesman, in my experience they are mostly clueless in the UK when it comes to buying electrical equipment, so I thought I would risk a little original research and ask whether any UK refdeskers have this feature on their TV and if so what is the make and model?--Shantavira|feed me 11:16, 21 June 2012 (UTC)
- Most retail salespeople are technically clueless. The best bet for now and long term is likely an PC with one or more DVB-T/C/S receiver cards and MythTV etc. The TV industry is quite dumbed down, computers is the game. Electron9 (talk) 14:37, 21 June 2012 (UTC)
- I have a fairly recent-ish Sony flatscreen - it doesn't have your desired feature. It overlays the EPG on top of the current picture, pretty much obscuring it. So scratch them off the list. You might be advised to wander into Currys, Dixons, John Lewis, etc., and see if you can get their salespeople to show you this function working on one of their TVs. --Tagishsimon (talk) 14:42, 21 June 2012 (UTC)
- Agreed, you can't trust salesmen, get them to show you the feature before you buy a model. I've had the same problem with cars, they either don't know how the "fancy buttons" work, just make it up as they go along, or outright lie to you. I test everything out myself before I agree to buy, and you should take your TV for a "test drive", too. StuRat (talk) 03:53, 22 June 2012 (UTC)
- My Samsung does this and so does a friends, do a google image search for 'Samsung EPG' and you can see what it looks like. Nanonic (talk) 06:39, 22 June 2012 (UTC)
- This answer doesn't apply to you because if your computer was running Linux you would know of this already. Yet, if you were running Linux, you would not have to worry about what TV to buy. You could watch TV on MythTV which provides a small live image of main channel as you require and since Linux allows you to have multiple desktops, you can quickly switch over to surfing the web (whilst still listening to the TV broadcast) and/or listen to music at the same time, and even do image processing, or what ever else you desire to do on your computer. If you like, you can have a larger image -of what ever size you so wish- and have it follow you around different desk tops. What to change channel at any point … No problem; just hit the numeric key number of the channel you now wish to watch. Come to think of it, I don't know why Microsoft hasn’t thought of coming out with a compact and cheap ($ 0.00) home-entertainment system like this. It would sell like hot pies. --Aspro (talk) 23:57, 22 June 2012 (UTC)
- It's a bread & butter company without visions, except in shareholder profit. As for Unix like BSD or Linux, getting any OS to handle video without interruption is the harder bit. Integration and being handle everything with remote control, device drivers etc.. is another thing to deal with. Electron9 (talk) 05:38, 24 June 2012 (UTC)
how much friction does water flowing through a (decent-sized) pipe undergo? if the pipe is long enough, would it not flow out, even vertically?[edit]
The question is how much friction water flowing through a (decent-sized) pipe undergoes, and whether, if the pipe is long enough, the water wouldn't even fall out the other side vertically, despite gravity and the other side being lower?
My thought process is as follows. It's very hot where I am right now. I noticed that the water that flowed out of the shower (set to cold water only) was still, after a moment, very very cold. Even standing a few feet away a draft of cool air hit me. If I had run it a very long time, it would have cooled my whole apartment off, which doesn't have an air conditioner in it. Of course, running water just for cooling is wasteful.
My next thought was: well, I'm not actually using it. Could someone hypothetically just run it, in a (clean) configuration that makes a big narrow wall of water with very large surface area, and simply (the water is still clean) feed it back into the system. Probably that would be possible. But it wouldn't be fair to the neighbors across the street (you could run it into their water intake) even if it's clean and sterile, because it's now warm. Why should they get warm water in the heat? (assuming they also want cold water.)
My next thought was: ah, but do you know who DOES want warm water: extreme latitudes where it's winter now. And vice versa. (If they cooled water, I would want it).
So here is my next thougth. Hypothetically, if we had a very long pipe between winter places that want warm water and summer places that want cold water, and we set the pipe to exactly the same altitude between the two places, then wouldn't the tiniest push (or vertical drop, like an aqeuaduct, which drops only a tiny amount over distance) of the end at either place cause the water to in the correspondeing direction? Since it doesn't actually take work (the physics concept) to keep something moving at the same momentum and altitude, less friction, the only thing that would stop such a scheme working for the next 100 years is if it were built is...water resistance in the pipe.
so, the question is: what IS that resistance? What stops my scheme from working, if anything? THe scheme is free cooling for summer climate and free heating for winter climate by running a water-based heat exchange between them with almost no work performed after building the aparatus. 84.3.160.86 (talk) 11:19, 21 June 2012 (UTC)
- by the time the water's reached the other side of the world it would have reached ambient temperature. Besides friction is considerable-try an experiment using drinking straws joined together, and blow down them. It would be easier to heat water through geothermal power or something. As for cooling, its generally reasonably cold a few meters under ground-which is why your water is cold in the first place. — Preceding unsigned comment added by 109.155.4.192 (talk) 12:17, 21 June 2012 (UTC)
- I'll answer the 2nd question first: The flow of water in a pipe is indeed determined by a form of friction - the viscosity of the water resists the movement of water against a surface. The flow of fluid (eg water) through a smooth pipe can be calculated by means of the Fanning-Darcy equation. There are two forms of Fanning-Darcy, one for simple laminar (stright line) flow, and one for turbulent flow. For laminar flow, the Fanning-Darcy equation is:-
- F = ( ΔP π D4 ) / ( 128 μ L )
- where F is the flow rate in m3/s; ΔP is the pressure difference along the length of the pipe in Pascals, π is 3.14.., D is the diameter of the pipe in meters, μ is the absolute viscosity of the fluid in mPa.s, L is the length of the pipe in meters. For turbulent flow, the formula is somewhat more complicated. To find whether you have laminar or turbulent flow, calculte the Reynolds Number:-
- Re = ( 4 ρ F ) / ( π μ D )
- If the Reynolds number is less than 2000, the flow will be laminar; if above 3000 it will be turbulent; between these 2 limits, some engineering judgement is required.
- All this means that (a) you will always get some flow no matter how long the pipe, and (b) the flow is inversley proportiona to pipe length, and proportional to the 4th power of the diameter (laminar flow; for turbulent, it is proportional to diameter raised to the power 19/7).
- As to the first question: Water walls are sometimes seen as a decorative feature in restaurants and hotel lobbies. It has some psychological effect in cooling perception, but the actual cooling effect is minimal, essentially being due to the conduction of heat from the air to the water over the water wall surfcae area. As far far more effective method of cooling is to evaporate the water, as the latent heat of vaporisation of water is very high. This is the principle being evaporative type airconditioners, often nicknamed "swampies" in the trade due to the use of wood shavings and the like to provide a very large surface area in a small volume.
- Don't forget that humans sweat - this is our built-in personal swampy, and makes you feel cool when in moving air, even if the air is not cool. You don't have to actually feel wet with sweat for this to work.
- Ratbone58.167.254.173 (talk) 12:31, 21 June 2012 (UTC)
- Also note that someone (or something, or some process ;-) has already put this system into production, and it seems to work well with only minor hickups so far! However, the tenants are playing with the thermostat and may possibly break it. --Stephan Schulz (talk) 13:57, 21 June 2012 (UTC)
- Good point, and well put too! Ratbone58.167.254.173 (talk) 15:40, 21 June 2012 (UTC)
- Also note that someone (or something, or some process ;-) has already put this system into production, and it seems to work well with only minor hickups so far! However, the tenants are playing with the thermostat and may possibly break it. --Stephan Schulz (talk) 13:57, 21 June 2012 (UTC)
- Water evaporation (usually spraying) is efficient for cooling and spreading legionella.. Electron9 (talk) 14:40, 21 June 2012 (UTC)
- Water evaporation does NOT spread legionella (bacteria can't hide in individual water molecules), however water spray or mist does spread it. However this must be seen in the proper context. When a large number "legionaires" who were at the Belevue-Stratford Hotel in 1976 got sick, the reason was investigated, and was shown to be an infection with a type of bacteria not previously known to science, sourced from the building aircon cooling towers. They named this "new" bug legionella. Soem years later, patients at an English hopital got sick and died. An investigation showed legionella was the cause, again sourced from airconditioning cooling towers. These and other incidents led to the rapid implementation of laws in various countries requiring owners of large buildings with aircon cooling towers to sample the water regularly and dose the water to keep the legionella count below arbitarily determined levels. It has since become very apparent that (a) almost invariably only people with compromised immune systems (such as hopital patients already sick and/or on prescribed drugs) are susceptable to legionella, and (b) legionella is ubiquitous in the enviroment - one of the most common forms of bacteria around. It can be argued that these laws are unnecessary - certainly they were enacted in haste without a true understanding of the issue. I worked for 6 years as a building manager responsible for several multi-storey sites with aircon cooling towers. Our aircon legionella count, due to dosing with bacteriacide per legal requirements, was lower than the count in water straight from the city water supply. Nobody legislates about legionella in domestic swampy airconditioners - it isn't necessary. Ratbone58.167.254.173 (talk) 16:09, 21 June 2012 (UTC)
- Not only do readers get impromtu tutorials here, we also have an encyclopedia with an article about Legionellosis. DriveByWire (talk) 23:25, 24 June 2012 (UTC)
- Water evaporation does NOT spread legionella (bacteria can't hide in individual water molecules), however water spray or mist does spread it. However this must be seen in the proper context. When a large number "legionaires" who were at the Belevue-Stratford Hotel in 1976 got sick, the reason was investigated, and was shown to be an infection with a type of bacteria not previously known to science, sourced from the building aircon cooling towers. They named this "new" bug legionella. Soem years later, patients at an English hopital got sick and died. An investigation showed legionella was the cause, again sourced from airconditioning cooling towers. These and other incidents led to the rapid implementation of laws in various countries requiring owners of large buildings with aircon cooling towers to sample the water regularly and dose the water to keep the legionella count below arbitarily determined levels. It has since become very apparent that (a) almost invariably only people with compromised immune systems (such as hopital patients already sick and/or on prescribed drugs) are susceptable to legionella, and (b) legionella is ubiquitous in the enviroment - one of the most common forms of bacteria around. It can be argued that these laws are unnecessary - certainly they were enacted in haste without a true understanding of the issue. I worked for 6 years as a building manager responsible for several multi-storey sites with aircon cooling towers. Our aircon legionella count, due to dosing with bacteriacide per legal requirements, was lower than the count in water straight from the city water supply. Nobody legislates about legionella in domestic swampy airconditioners - it isn't necessary. Ratbone58.167.254.173 (talk) 16:09, 21 June 2012 (UTC)
- Water evaporation (usually spraying) is efficient for cooling and spreading legionella.. Electron9 (talk) 14:40, 21 June 2012 (UTC)
Science of weight loss and fitness[edit]
Can anyone direct me to some good source of science on the subject? Also I would like to ask a couple of questions, Are raw eggs any better than cooked eggs? Should you take protein just before working out or you just need to have them in your diet? Bastard Soap (talk) 14:09, 21 June 2012 (UTC)
- The science on this is still very much uncertain, you could search in some scientific journals, like here:
- Count Iblis (talk) 16:03, 21 June 2012 (UTC)
- Unless they are burnt, proteins are broken down into the same amino acids whether they are cooked or not. Cooking has the huge benefit of killing potentially dangerous bacteria. So long as you do not burn the eggs you will not lose any nutritional values. Boil or scramble them. If convenience matters, one or two eggs scrambled in a bowl and then microwaved for about a minute (until dry, you may need to stir once) will come out quite nice. Since just one egg already exceeds a normal person's daily recommended cholesterol you might look into other sources like whey protein. Consult a professional. μηδείς (talk) 16:35, 21 June 2012 (UTC)
- My understanding is that those cholesterol recommendations are out of date, because of considerable data showing that dietary cholesterol has only a weak influence on the buildup of cholesterol in arteries. But in any case none of this protein stuff really matters except to extreme bodybuilders, and you certainly don't need major protein supplementation to achieve weight loss. Looie496 (talk) 18:04, 21 June 2012 (UTC)
It's so strange to not have definite answers about this stuff, it's what most people are most interested in and the science is very lacking.Bastard Soap (talk) 22:04, 21 June 2012 (UTC)
- My own experience suggests that some of the fundamentals of nutritional science are totally flawed like the link between calorie intake and weight. On theoretical grounds, you would not expect there to be a strong link, because if you increase your calorie intake, you'll eventually reach a dynamical equilibrium where you burn the same amount of calories as you are consuming. That your weight should be linked to that dynamical equilibrium is not clear at all. The argument that if you eat more than you burn, you'll store more fat, is a red herring, as it doesn't address the issue of dynamical equilibrium.
- People who are naturaly thin have been tested in an experiment where they were given 5000 Kcal/day to eat. Their weights didn't increase by a lot at all.
- Then my own experience also contradicts the idea of there being a strong link between calorie intake and weight. I consume about 3500 Kcal/day, yet I only weigh about 60 kg. Some of my obese friends and family members eat a lot less than I do, but they don't eat healthy foods. This suggests that what matters is the vitamins and the minerals you consume. The body needs these to burn carbohydrates and fat efficiently. Count Iblis (talk) 02:04, 22 June 2012 (UTC)
- Perhaps excess calories go undigested in some, but I can't believe your metabolic rate goes up tenfold if you eat ten times as many calories. You'd burst into flames. StuRat (talk) 03:34, 22 June 2012 (UTC)
- I presume the excess calories are turned into bacteria, and then pooped out. Your gut microbiome is pretty good at getting rid of most of the nutrition you couldn't absorb yourself. Regarding the question of protein intake, even in the case of bodybuilders there is only so much protein your body can process in a day. In fact, the average american's diet contains more than enough protein for even a weightlifting regimen. All those protein supplements for weight building may as well be snake oil (along with most nutritional supplements for that matter). Someguy1221 (talk) 07:44, 22 June 2012 (UTC)
- Perhaps excess calories go undigested in some, but I can't believe your metabolic rate goes up tenfold if you eat ten times as many calories. You'd burst into flames. StuRat (talk) 03:34, 22 June 2012 (UTC)
As far as I know when they list calories that's the total ammount not the digestible ammount, they burn the shit and tell you how much power it gave them. One single experiment means very little it could easily be biased, it becomes a truth when a lot of scientists replicate the results. The dynamic equilibrium makes little sense to me and is easily disproven by eating only pizzas for a week. Sturat I think you're closer to the truth and some people are less efficient.Bastard Soap (talk) 07:35, 22 June 2012 (UTC)
- Just eat a bit less every day. You don't starve to death if you eat 100 Kcal/day less and keep your activity level the same (or increase it a bit to make sure that during exercise you still burn the same amount of calories or more than you used to, despite having lost some weight). Yet, the simplistic models used for weight gain would predict that after 10 years, you should have lost 45 kg body weight. Clearly, that won't happen, but that means that the body maintains the energy balance by regulating the metabolic rate. But that implies that you won't keep on gaining weight if you eat 100 Kcal per day more, day after day. Count Iblis (talk) 18:11, 22 June 2012 (UTC)
gauss and gauss meters[edit]
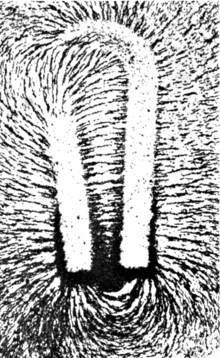
Could someone please explain to me in very simple terms (I am not a physicist or a scientist, but an archaeologist) what a gauss meter measures and how to interpret the results I get from it? I am not really clear on what gauss represents, either. Thank you. — Preceding unsigned comment added by 184.9.192.187 (talk) 14:15, 21 June 2012 (UTC)
- A gauss meter measures the strength of a magnetic field. See Gauss (unit). Red Act (talk) 14:39, 21 June 2012 (UTC)
- Please see Magnetic survey (archaeology). In very simple terms, buried objects distort the Earth's magnetic field at the surface. By measuring the field at a large number of points, you can create a map showing where the field varies and by how much. --Heron (talk) 18:05, 21 June 2012 (UTC)
- See also gaussmeter. Red Act (talk) 19:01, 21 June 2012 (UTC)
Have you ever looked at iron filings lining up like the Earth's magnetic field due to a magnet? Where the lines are more spread out the magnetic field is weaker. Where the lines are more densely packed the field is stronger. The Gauss meter is measuring the (invisible to you) density of those lines. μηδείς (talk) 03:06, 22 June 2012 (UTC)
What feels like a throat but isnt'?[edit]
I've been practising martial arts for a while now and I would like to get a good test of my kill strikes. Are there any common fruits or foods or other common objects which have a similar consistency and strength to the human throat or solar plexus?Bastard Soap (talk) 14:29, 21 June 2012 (UTC)
- If you don't get queasy easily, get a dead pig, they are somewhat close to us biologically - skin, bones, meat. The carcass is gonna get ripe on you though. Compare that feel with melons and such to find something that feels similar - or at least looks cool when you make a mess of a melon in front of your friends. A more practical way is to look in your local yellow pages for companies that sell martial arts equipment; they can sell you a practice dummy that won't break or turn rancid. 88.114.124.228 (talk) 17:50, 21 June 2012 (UTC)
- 'Tis should be a strange world if we should feel comfortable with answering "I would like to get a good test of my kill strikes."
- I've removed the question, and OP you should obviously give this some some thought thought before you get you get into any trouble. --80.99.254.208 (talk) 19:37, 21 June 2012 (UTC)
They give some advice in Human Wrecking Balls. Count Iblis (talk) 19:47, 21 June 2012 (UTC)
Wow, I did not expect this from wikipedia. All of martial arts is about making yourself a weapon and to be used only if absolutely necessary, I have no intention of using it except if I'm attacked by some rabid dog, I just want to know if I'm able to. If I wanted to kill someone I'd get a knife jesus.Bastard Soap (talk) 21:22, 21 June 2012 (UTC)
- Veiled legal threats, ad hominem and crystall ballery aside, I don't see how this question violates policy, or how removing it is appropriate. I've replaced it. 203.27.72.5 (talk) 22:11, 21 June 2012 (UTC)
- I second the meat hypothesis. Vespine (talk) 23:10, 21 June 2012 (UTC)
- Do you mean if a rabid dog attacks you, you intend to strike the throat of the nearest human just to prove you were able to kill someone by a throat strike before you potentially lose your life? Nil Einne (talk) 05:17, 22 June 2012 (UTC)
Grapefruit. μηδείς (talk) 23:28, 21 June 2012 (UTC)
I imagine dog throats are not that different.Bastard Soap (talk) 08:27, 22 June 2012 (UTC)
- I suggest WP:DNFTT. --Saddhiyama (talk) 08:34, 22 June 2012 (UTC)
- But why would you ask for human throats if what you actually want to practice on is dog throats? Nil Einne (talk) 05:35, 24 June 2012 (UTC)
You could train your dog to attack you and then claim self defense when you kill it. good luck165.212.189.187 (talk) 15:40, 22 June 2012 (UTC)
It sounds like none of you have ever taken martial arts, being able to kill and actually killing or wanting to kill are very seperate thingsBastard Soap (talk) 10:41, 23 June 2012 (UTC)
- Actually I did when I was younger, but never desired to learn how to kill. In fact I think if I'd told my instructor I wanted to learn to kill, I probably would have been kicked out of the class. I would note many martial arts concentrate on self defence, particularly the ability to disable, at least sufficiently to escape since being able to kill is often not of particular use. Nil Einne (talk) 05:35, 24 June 2012 (UTC)
Robots[edit]
Could you make a robot with real human skin? 176.250.196.132 (talk) 15:26, 21 June 2012 (UTC)
- Well you can get human skin book covers, so I don't see why that shouldn't be extended to robots. Access for maintenance would be a bit tricky, and of course the skin would be dead and not very convincing if you're trying to make an android.--Shantavira|feed me 15:59, 21 June 2012 (UTC)
- But can you make an android with living human skin. 176.250.196.132 (talk) 17:46, 21 June 2012 (UTC)
- In principle, yes. But you would have to work out a way to supply it with all the necessary nutrients and complex molecules, which means adding an artificial bloodstream and a lot of very complex biochemical fabricating machinery. I can't imagine any possible benefit that would be worth the enormous effort. Looie496 (talk) 17:53, 21 June 2012 (UTC)
- Oh, it's essential. Non-living matter can't travel backwards in time. Apparently, unless it's covered by living matter — that part never seemed to be explained in detail, but anyway it was a nice excuse to show a (fairly chaste) nude of Summer Glau on broadcast TV in prime time. --Trovatore (talk) 19:11, 21 June 2012 (UTC)
- Because that worked so well the first time! —Tamfang (talk) 03:40, 25 June 2012 (UTC)
- Uh-oh. Not only has Skynet acheived self-awareness, it's asking questions on the Wikipedia Ref Desk.FlowerpotmaN·(t) 19:16, 21 June 2012 (UTC)
- Oh, it's essential. Non-living matter can't travel backwards in time. Apparently, unless it's covered by living matter — that part never seemed to be explained in detail, but anyway it was a nice excuse to show a (fairly chaste) nude of Summer Glau on broadcast TV in prime time. --Trovatore (talk) 19:11, 21 June 2012 (UTC)
- In principle, yes. But you would have to work out a way to supply it with all the necessary nutrients and complex molecules, which means adding an artificial bloodstream and a lot of very complex biochemical fabricating machinery. I can't imagine any possible benefit that would be worth the enormous effort. Looie496 (talk) 17:53, 21 June 2012 (UTC)
- But can you make an android with living human skin. 176.250.196.132 (talk) 17:46, 21 June 2012 (UTC)
Would it be possible that in the distant future, it wouldnt be so complex, with advancing biological research? 176.250.196.132 (talk) 21:54, 21 June 2012 (UTC)
- Well, you can already remove human bone and replace it with metal. It's not such a big leap to remove all human bones and replace them with synthetic materials, though it would be much more technically difficult (you might exceed the $6million budget). Then you could look at doing a brain transplant, but instead of a brain, install some sort of computer control device. I'd say the technology will be that advanced relatively soon, but unfortunately, the we've long since past the times where that might be ethically possible. 203.27.72.5 (talk) 22:03, 21 June 2012 (UTC)
- It is uncertain whether the above poster means "we've long passed the times when" or "we're long past the times when", either of which is grammatically possible. DriveByWire (talk) 23:11, 24 June 2012 (UTC)
- Even such a limited thing as bones illustrates the difficulty. Replacing one bone is relatively minor, but bone marrow is what generates blood cells, so replacing all the bones would leave you with mere plasma for blood. Looie496 (talk) 22:26, 21 June 2012 (UTC)
- You could use perflourocarbon blood substitutes. HominidMachinae (talk) 23:59, 21 June 2012 (UTC)
- Even such a limited thing as bones illustrates the difficulty. Replacing one bone is relatively minor, but bone marrow is what generates blood cells, so replacing all the bones would leave you with mere plasma for blood. Looie496 (talk) 22:26, 21 June 2012 (UTC)
- A much lower level of technology could provide fake skin indistinguishable from the real thing. StuRat (talk) 03:06, 22 June 2012 (UTC)
- Sorry StuRat, but the laws of time travel cannot be fooled. 203.27.72.5 (talk) 03:58, 22 June 2012 (UTC)
- Currently, no. However, futurists like Ray Kurzweil have imagined our technology reaching the Picotechnology scale. If and when we can design at the atomic or subatomic level, our current distinctions of Human being, robot and android would become moot. Would you describe a dna and protein based, laboratory grown intelligent organism which looks human, and has an enhanced brain using quantum computing a human (ie has perhaps a soul), a robot (a machine designed to do work for us), or an android (a device designed to mimic the human form). for that matter, will WE still be human. Greg Bear says we wont be recognizably human in the near future. Practically speaking, the cost and effort involved to create a synthesized organism with elements of a machine, with human skin and the supporting biological systems that would keep the skin alive and functional (sweat glands, blood flow and oxygen transport, nerves connected to a brain or computer, etc), make it unlikely we will be making a terminator type "robot with skin" at any point. too many other things to do at that point of our technological evolution. What purpose would it serve? skin would make it more vulnerable to the conditions that we usually design robots to handle (working conditions not ideal for humans).(mercurywoodrose)50.193.19.66 (talk) 17:14, 25 June 2012 (UTC)
- An android would be useful for many purposes, such as a nannybot to care for the kids. If you gave them a nanny that looked like Robby the Robot, it wouldn't be very acceptable to your average toddler (and might cause a potty training failure when they first see it). One that looks human would be better. StuRat (talk) 18:43, 25 June 2012 (UTC)
- I can imagine assigning a nanobot as understudy to each cell in your body, or at least each nerve cell; when the cell dies, the understudy takes over its function, interfacing with its neighbors. Eventually you're all artificial but the difference may be hard to tell. In such a scenario, I'd want to keep my skin as long as practical, for social reasons and for the pleasures of tactility. —Tamfang (talk) 18:13, 25 June 2012 (UTC)
Refractive index[edit]
What kind of material has the highest refractive index?--Jsjsjs1111 (talk) 15:52, 21 June 2012 (UTC)
- When asking what material has the highest refractive index, it's necessary to specify what wavelength you're interested in, because the refractive index varies widely with the wavelength, in a non-uniform way. The link Zzubnik gives refers to a material that has a maximum refractive index of 38.6 at frequencies near 0.3 THz, which corresponds to a wavelength (in vacuum) of about 1 mm. However, standard refractive index measurements are taken with light with a wavelength in vacuum of 589 nm (the sodium D line), which is more than a thousand times smaller. The highest refractive index at that wavelength listed in our list of refractive indices, at least, is 4.01, for germanium. Red Act (talk) 16:59, 21 June 2012 (UTC)
- For minerals in light you can check out rutile (2.9) and hematite (3.22). Around absorption lines you can get some rapidly changing high numbers. Also for artificial substances look at Barium titanate and Lead zirconate titanate which will have very high values at longer wavelengths. Graeme Bartlett (talk) 21:35, 21 June 2012 (UTC)
I see. Thank you very much!--Jsjsjs1111 (talk) 07:15, 22 June 2012 (UTC)
Creep (deformation)[edit]
At my job, I'm often moving around big stacks of boxes (just a few at a time; I don't have any power machinery to help me) that are full of newspapers. As you can imagine, the stacks tend to lean if the boxes aren't placed properly, and improper placement of boxes toward the bottom of stacks can result in damage. In some stacks, there are different sizes of boxes, with the wider ones sitting on top of the narrower ones, and the wider ones are damaged — look at this:
__________________________ |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| |________________________| | ____________________ | \/ | | \/ |__________________|
The edges of the bottom of the lowest wide box have nothing under them, so the lowest wide box is gradually squished and its edges splay out around the top of the not-so-wide box. I've never seen this happen instantly — I can put a big stack of heavy wide boxes on top of a single not-so-wide box, and they all just sit there without problems, but if I come back a few weeks later, there is substantial damage even if nobody else touch the stack. Is all of this an example of Creep (deformation)? I don't understand the article well enough to say either yes or no. Nyttend (talk) 21:30, 21 June 2012 (UTC)
- It's analygous to creep, but not exactly the same thing. Creep is essentially caused by stresses in metals having an effect on the solid state diffusion through the crystal lattice, so as to increase relaxation. That's why being close to the melting point dramatically increases creep. In this case I think the friction between the sheets of newspaper prevents instant relaxation but as it sits and goes through a few day/night cycles of heating and cooling the sheets will move a tiny amount accross each other as the aggregate structure deforms around the smaller box placed underneath. 203.27.72.5 (talk) 21:56, 21 June 2012 (UTC)
- (EC)It's an example of slow permanent deformation, so it probably can be considered an example of creep. Our article won't help you though with mechanism by which it occurs as the cardboard (I'm assuming that's the material that the boxes are made from) won't deform like a metal or ice. No doubt there are many papers written on this topic and here's the sort of apparatus that can be used to test the creep behaviour of corrugated cardboard boxes[2]. Mikenorton (talk) 22:03, 21 June 2012 (UTC)
- And here's a paper on just this sort of behaviour [3]. Mikenorton (talk) 22:09, 21 June 2012 (UTC)
- What a great question and reference/answer! SemanticMantis (talk) 00:00, 22 June 2012 (UTC)
- Thanks very much for the help! Yes, they're corrugated cardboard boxes; sorry that I forgot to mention that before. They're normally full, although for some newspaper titles we don't quite have enough to fill a box, and those ones (not surprisingly) appear to be more vulnerable to deformation — they sometimes exhibit comparable behavior in the middle of a stack, when they have equally sized boxes both above and below them. In such cases, the sides splay out and the boxes on top will appear to have sunken into the partly empty box, squishing the partly empty box's lid in the process. I think I'll read the Georgia Tech paper if I get the chance. Nyttend (talk) 14:54, 22 June 2012 (UTC)
- What a great question and reference/answer! SemanticMantis (talk) 00:00, 22 June 2012 (UTC)
- And here's a paper on just this sort of behaviour [3]. Mikenorton (talk) 22:09, 21 June 2012 (UTC)
Why exactly does water stop bullets?[edit]
Hello. Mythbusters recently showed a clip from the time they tested shooting bullets into water, and watched how they disintegrated a few feet in. Apparently they blamed this stopping power on the "incompressibility of water". Nevermind water is not exactly "incompressible" (just very hard to compress); does saying "water is (nearly) incompressible" (or something similar) really explain the bullet-stopping power? Or what exactly would you have to say about water to explain why it's so effective against bullets? Thanks in advance. --Kreachure (talk) 23:28, 21 June 2012 (UTC)
- well, nothing is exactly incompressible. --Trovatore (talk) 23:42, 21 June 2012 (UTC)
There are a few factors at play. First of all, many modern bullets for civilian use are what they call frangible, or meant to break up or mushroom on impact. This is so that they transfer their energy more quickly and more completely into the target. Virtually every hunting round falls into this category and even non-frangible civilian ammo is jacketed soft-point at best. Only the military uses fully jacketed rounds that do not deform normally in use. The water causes the round to start to mushroom or break apart (depending on the type of round) and this destroys its aerodynamics (hydrodynamics?) and causes it to lose speed more rapidly due to resistance. In addition the density of the water rapidly saps kinetic energy from the bullet. Remember most bullets are VERY fast but actually don't have all that much in terms of actual momentum because they typically weigh comparatively very little. if you take into account the fact the round is being pushed out of shape it can lose speed in as little as a foot or two of water. the rounds that perform best in water are smaller, very fast and smooth-nosed rounds like the 9x19mm Parabellum (as proven on mythbusters) but if the round is too tiny it will fracture (as mythbusters saw with the 5.56mm NATO round). There are special rounds designed for underwater use, in use by Russia notably in a modified AK-47 frame. The rounds are designed like flechettes or darts with a very low cross-section and long length and designed for hydrodynamics. I have NO basis for this but logic would indicate there may also be a hydrodynamic influence, if the bullet created a low-pressure cavitation behind it, that would further slow it. HominidMachinae (talk) 23:45, 21 June 2012 (UTC)
- To further what Hominid said, civilian ammunition is designed to break up because that causes the most damage to flesh. It might seem counter intuitive that military ammunition has a key design element that inhibits it from damaging flesh, but that's an historical artifact from the hague convention. Anyways, since flesh is mostly water, the round behaves in water much the same way as it does in flesh; its cross-section expands and it breaks up. 203.27.72.5 (talk) 01:12, 22 June 2012 (UTC)
- As to what physical processes cause it to break up, when the bullet is travelling though air, it has very little to resist its motion. When the tip of the bullet makes contact with water/flesh, the resistance is much greater and it slows very quickly (it may still be travelling quite fast, but it's rate of deceleration is very high). This sends a shockwave through the length of the bullet which causes it to break apart. The shock wave travels at the speed of sound (in the metal that the bullet is made out of) with respect to the bullet. Since the bullet itself may be going faster than the speed of sound, the shockwave may not reach the back of the bullet until it's already travelled more that it's own length into the flesh. If the bullet has a soft nose, the lead at the front will also be deformed and material will be forced down into the nose and cleave it apart leading to the mushroom effect. If it has a hollow nose, this effect can be even stronger.
- The flechette style rounds that Hominid mentioned are made of hard metals that don't break up easily (such as steel alloys) and as he said, their small cross section aids them in getting through flesh without being clogged up with flesh in the nose (like a hypodermic syringe). You could say that the fact that air is compressible is what leads to it not resisting the bullet's motion. You could also just say that its density is so much lower that there is less mass to heave out of the way. The fact that water/flesh is compressible is important in the damage caused by bullets also; a compression wave originiates at the point of impact and propagates through the body potentially causing damage to organs far away from the bullet's path. 203.27.72.5 (talk) 01:22, 22 June 2012 (UTC)
- Excellently put, I'd also like to point out that military ammo has other constraints on it other than maximum flesh damage. A full metal jacket round is kinder on barrels especially when firing fully automatic (important if you want your rounds to be compatible with your squad support weapons), it also has greater armor penetration. To top it all off the noses of hollow points (unless capped) have problems feeding in some automatic mechanisms, that was the reason the smooth-nosed 9mm Parabellum was invented to begin with. Some self-defense experts advise using revolvers for defense and concealed carry for that very reason, you can carry a mushrooming round without risking a feed jam, and if a round misfires you can just pull the trigger again and rotate the cylinder. HominidMachinae (talk) 01:37, 22 June 2012 (UTC)
Not to trivialize an interesting discussion but, could you then say in simple terms that water can stop bullets "because water is much denser than air, and a bullet encounters so much resistance when in water that it can't clear a path and breaks apart"? I hope I'm not oversimplifying too much. Apart from this, the shockwave aspect that was mentioned I find very hard to believe. Do you mean that things that are slowed very fast are destroyed by the resulting shockwave, rather than by the "being slowed" itself? If this is the case, could you provide other examples where this happens? --Kreachure (talk) 02:04, 22 June 2012 (UTC)
- I'm not sure that you're grasping the concept of a shockwave. The nose of the bullet slows first. The particles (of Pb) that comprise the nose slow down and the bonds to the particles immediately behind them are compressed. This compression continues in a wave through to the back of the bullet. At points such as lattice defects the bond compression may be enough to break the bonds and consequently break the bullet apart. You can't distinguish the shockwave caused by the deceleration from the "being slowed" itself. 203.27.72.5 (talk) 02:28, 22 June 2012 (UTC)
- I don't think you're oversimplifying much, except that the mechanism of the bullet mushrooming is vital to understanding the issue here, and why some bullets might NOT be affected that much by water. HominidMachinae (talk) 02:30, 22 June 2012 (UTC)
- A full Coke can certainly can stop relatively low speed projectiles such as air rifle pellets, or projectiles with poor aerodynamic characteristics like shotgun pellets. It might stop a .22 or .177. It would have a better chance if you shot it length ways down from the spout. It won't stop a .50cal though. Not even length ways. 203.27.72.5 (talk) 02:34, 22 June 2012 (UTC)
Speed of sound in lead is 1 km/s, while pistol rounds travel half that fast. Ergo no shockwave inside the bullet. Hcobb (talk) 02:42, 22 June 2012 (UTC)
- Your conclusion does not follow from your premise. The abrupt change in velocity is what constitues the shockwave, not the velocity itself. 203.27.72.5 (talk) 02:50, 22 June 2012 (UTC)
- Hcobb is right. Most bullets do not travel fast enough to experience internal shock waves on impact. The bullets experience an impact (mechanics) resulting in a shock (mechanics) that creates an extreme compression wave in the bullet that does tear the bullet apart. However, since the bullets are usually traveling slower than the speed of sound in lead, the resulting wave is merely a compression wave and not a shock wave. An actual shock wave requires a disturbance that propagates faster than the internal sound speed, which will not occur at typical bullet speeds. This distinction is technical, but not horribly relevant. The subsonic compression waves created by a bullet's impact are still more than enough to rip it apart. Dragons flight (talk) 05:28, 22 June 2012 (UTC)
Um, water stops bullets for the same exact reason human the human body stops bullets. It's mostly made of water. μηδείς (talk) 02:55, 22 June 2012 (UTC)
- To rephrase that slightly, water stops bullets for the same reason that bullets stop people. Hcobb (talk) 13:35, 22 June 2012 (UTC)
- I guess I've never seen a "modern bullet" which shatters when it hits water or anything else. I've fired rifle and pistol bullets in years past which were basically a streamlined piece of lead. If "modern bullets" feel "shock waves" which cause them to fall apart when they hit water, then how come ballistics testing of a pistol starts with firing it into a water tank, after which the intact bullet is examined microscopically for striations which can match it to crime scene bullets? Certainly bullets extracted from someone's body may be mushroomed or distorted, but not always shattered into fragments. I've heard of the "frangible rounds" being used by sky marshals, so they don't shoot holes in a pressurized airplane, or so a bullet does not pass through the "bad guy" and hit a "good guy," but if I were shooting a game animal for the pot, I would rather have one bullet to remove than bits of shattered bullet fragments. If I were a soldier or cop shooting at an opponent, I would not want to have fragile bullets which shattered on contact with something, while they had non-shattering bullets which could go through a door or car and still kill me. Edison (talk) 14:51, 22 June 2012 (UTC)
- Where are you shooting your game? You don't need to remove the bullet from the brain, unless you want to eat that and risk prion infection. And not too many people have a taste for heart for game animals. In forensics, the bullets are shot into water containing a surfactant to minimize surface tension and at a shallow angle. It can also take many firings to produce an intact specimen even under those circumstances. 203.27.72.5 (talk) 23:02, 22 June 2012 (UTC)
- See this eBook for an excellent explanation of what goes on as a bullet impacts flesh, water and other surfaces. 203.27.72.5 (talk) 23:13, 22 June 2012 (UTC)
- The bullets break up and they try to id the fragments apparently[4]. Or some suggest they use low-velocity loads in water tanks. Rmhermen (talk) 23:38, 22 June 2012 (UTC)
- I said SOME frangible bullets are designed to break up, typically some kinds of anti-personnel loads and training rounds, far more common is the mushrooming kind (jacketed hollowpoint, hollowpoint, wadcutter, semi-wadcutter, AET, AET composite). There are fragmenting rounds like Glaser, Spartan, Equalloy and the potentially mythical mercury). HominidMachinae (talk) 17:38, 23 June 2012 (UTC)
- Where are you shooting your game? You don't need to remove the bullet from the brain, unless you want to eat that and risk prion infection. And not too many people have a taste for heart for game animals. In forensics, the bullets are shot into water containing a surfactant to minimize surface tension and at a shallow angle. It can also take many firings to produce an intact specimen even under those circumstances. 203.27.72.5 (talk) 23:02, 22 June 2012 (UTC)


