User:Scarey808/sandbox
| Squalene synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 2.5.1.21 | ||||||||
| CAS no. | 9077-14-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| farnesyl-diphosphate farnesyltransferase 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FDFT1 | ||||||
| NCBI gene | 2222 | ||||||
| HGNC | 3629 | ||||||
| OMIM | 184420 | ||||||
| RefSeq | NM_004462 | ||||||
| UniProt | P37268 | ||||||
| Other data | |||||||
| EC number | 2.5.1.21 | ||||||
| Locus | Chr. 8 p23.1-p22 | ||||||
| |||||||
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme in the isoprenoid biosynthetic pathway. Squalene synthase catalyzes a two-step reaction in which two identical molecules of farnesyl diphosphate (FPP) are converted into squalene.[2]

It has been described as the first dedicated enzyme of sterol (i.e., cholesterol, etc.) synthesis, since the squalene formed by it is exclusively routed into various sterols via a complex, multi-step pathway.
Diversity[edit]
Squalene synthase is considered to be an enzyme of eukaryotes or advanced organisms, although at least one prokaryote has been shown to possess a functionally similar enzyme.
In terms of structure and mechanics, squalene synthase most closely resembles phytoene synthase, which serves a similar role in many plants in the elaboration of phytoene, a precursor of many carotenoid compounds. (Carotenoids are the colorful pigments present in most vegetables.)
Interactive pathway map[edit]
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
Structure[edit]
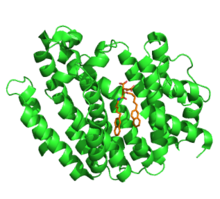
Squalene synthase (SQS) is a 47-kDa enzyme localized to the membrane of the endoplasmic reticulum.[3] SQS is anchored to the membrane by a C-terminal membrane-spanning domain.[4] The N-terminal catalytic domain of the enzyme protrudes into the cytosol.[2] The crystal structure of human SQS was determined in 2000, and revealed that the protein was comprised of thirteen alpha-helices. The enzyme is folded into a single domain, characterized by a large central channel. The active sites of the two half-reactions catalyzed by SQS are located within the channel. One end of the channel is open to the cytosol, whereas the other end forms an enclosed hydrophobic pocket. Squalene synthase contains two conserved aspartate-rich sequences, which are believed to participate in the catalytic mechanism.[3]
Mechanism[edit]
Squalene synthase (SQS) converts two molecules of farnesyl pyrophosphate (FPP) into squalene via a two-step mechanism. FPP is a soluble C15 allylic compound, whereas squalene is an insoluble, C30 isoprenoid. [2][5] This reaction is a head-to-head terpene synthesis, because the two FPP molecules are both joined at the C1 position.[6][7] The reaction mechanism requires a divalent cation, often Mg2+, to facilitate pyrophosphate release [8].
FPP 1'-1 condensation[edit]
In the first half-reaction, two identical molecules of farnesyl pyrophosphate (FPP) are bound to squalene synthase (SQS) in a sequential manner.[9] The pyrophosphate group is cleaved from one molecule of FPP, designated as the donor prenyl. The resulting allylic carbocation reacts with the C2,3 double bond of the acceptor FPP in a 1',2,3 prenyl transferase reaction, forming the stable intermediate presqualene pyrophosphate (PSPP).[7] The 1'-1 condensation of the two FPP molecules releases a pyrophosphate and a proton (H+). The PSPP created remains associated with squalene synthase for the second reaction. [3][8]
PSPP rearrangement and reduction[edit]
In the second half-reaction of SQS, the intermediate presqualene pyrophosphate (PSPP) moves to a second reaction site within SQS. Keeping PSPP in the central channel of SQS is thought to protect the reactive intermediate from reacting with water. [3] PSPP is rearranged and reduced using NADPH to produce the final product, squalene. SQS released squalene into the membrane of the endoplasmic reticulum.[2] This reaction also produces pyrophosphate, H+, and NADP+.[8]
Biological Function[edit]
Squalene synthase (SQS) is an enzyme participating in the isoprenoid biosynthetic pathway. SQS synthase catalyzes the branching point between sterol and nonsterol biosynthesis, and commits farnesyl pyrophosphate (FPP) exclusively to production of sterols.[2] An important sterol produced by this pathway is cholesterol, which is used in cell membranes and for the synthesis of hormones.[10] SQS competes with other enzymes for use of FPP, since it is a precursor for a variety of terpenoids. Decreases in SQS activity limit the flux of FPP to the sterol pathway, and increase the production of nonsterol products.[11]
Development of squalene synthase knockout mice has demonstrated that loss of squalene synthase is lethal, and that the enzyme is essential for development of the central nervous system.[12]
Disease Relevance[edit]
Squalene synthase is a target for the regulation of cholesterol levels. Increased expression of SQS has been shown to elevate cholesterol levels in mice.[12] Therefore, inhibitors of SQS are of great interest in the treatment of hypercholesterolemia and prevention of coronary heart disease (CHD).[13]
Squalene synthase inhibitors[edit]
Squalene synthase inhibitors have been shown to decrease cholesterol synthesis, as well as to decrease plasma triglyceride levels.[10][14] SQS inhibitors may provide an alternative to HMG-CoA reductase inhibitors (statins), which have problematic side effects for some patients. [15]
Other squalene synthase inhibitors that have been investigated for use in the prevention of cardiovascular diseases include lapaquistat, Zaragozic acid, and RPR 107393. [16] [17] Lapaquistat is no longer being investigated for clinical use.
It has also been suggested that variants in this enzyme may be part of a genetic association with hypercholesterolemia.[18]
Squalene synthase homolog inhibition in Staphylococcus aureus is currently being investigated as a virulence factor-based antibacterial therapy.[19]
References[edit]
- ^ Ichikawa, M.; Yokomizo, A.; Itoh, M.; Sugita, K.; Usui, H.; Shimizu, H.; Suzuki, M.; Terayama, K.; Kanda, A. (2011). "Discovery of a new 2-aminobenzhydrol template for highly potent squalene synthase inhibitors". Bioorganic & Medicinal Chemistry. 19 (6): 1930–1949. doi:10.1016/j.bmc.2011.01.065. PMID 21353782.
- ^ a b c d e T. R. Tansey & I. Shechter (2000). "Structure and regulation of mammalian squalene synthase". Biochimica et Biophysica Acta. 1529 (1–3): 49–62. doi:10.1016/s1388-1981(00)00137-2. PMID 11111077.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ a b c d J. Pandit, D. E. Danley, G. K. Schulte, S. Mazzalupo, T. A. Pauly, C. M. Hayward, E. S. Hamanaka, J. F. Thompson & H. J. Jr Harwood (September 2000). "Crystal structure of human squalene synthase. A key enzyme in cholesterol biosynthesis". The Journal of Biological Chemistry. 275 (39): 30610–30617. doi:10.1074/jbc.M004132200. PMID 10896663.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ S. M. Jennings, Y. H. Tsay, T. M. Fisch & G. W. Robinson (July 1991). "Molecular cloning and characterization of the yeast gene for squalene synthetase". Proceedings of the National Academy of Sciences of the United States of America. 88 (14): 6038–6042. doi:10.1073/pnas.88.14.6038. PMC 52017. PMID 2068081.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ T. R. Tansey & I. Shechter (2001). "Squalene synthase: structure and regulation". Progress in Nucleic Acid Research and Molecular Biology. 65: 157–195. doi:10.1016/s0079-6603(00)65005-5. ISBN 9780125400657. PMID 11008488.
- ^ Poulter, C. Dale (1990). "Biosynthesis of non-head-to-tail terpenes. Formation of 1'-1 and 1'-3 linkages". Accounts of Chemical Research. 23 (3): 70–77. doi:10.1021/ar00171a003. ISSN 0001-4842.
- ^ a b Fu-Yang Lin, Chia-I. Liu, Yi-Liang Liu, Yonghui Zhang, Ke Wang, Wen-Yih Jeng, Tzu-Ping Ko, Rong Cao, Andrew H.-J. Wang & Eric Oldfield (December 2010). "Mechanism of action and inhibition of dehydrosqualene synthase". Proceedings of the National Academy of Sciences of the United States of America. 107 (50): 21337–21342. doi:10.1073/pnas.1010907107. PMC 3003041. PMID 21098670.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b c E. Beytia, A. A. Qureshi & J. W. Porter (March 1973). "Squalene synthetase. 3. Mechanism of the reaction". The Journal of Biological Chemistry. 248 (5): 1856–1867. doi:10.1016/S0021-9258(19)44269-5. PMID 4348553.
{{cite journal}}: CS1 maint: date and year (link) - ^ K. A. Mookhtiar, S. S. Kalinowski, D. Zhang & C. D. Poulter (April 1994). "Yeast squalene synthase. A mechanism for addition of substrates and activation by NADPH". The Journal of Biological Chemistry. 269 (15): 11201–11207. doi:10.1016/S0021-9258(19)78111-3. PMID 8157649.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b A. P. Kourounakis, M. G. Katselou, A. N. Matralis, E. M. Ladopoulou & E. Bavavea (2011). "Squalene synthase inhibitors: An update on the search for new antihyperlipidemic and antiatherosclerotic agents". Current Medicinal Chemistry. 18 (29): 4418–4439. doi:10.2174/092986711797287557. PMID 21864285.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eric M. Paradise, James Kirby, Rossana Chan & Jay D. Keasling (June 2008). "Redirection of flux through the FPP branch-point in Saccharomyces cerevisiae by down-regulating squalene synthase". Biotechnology and Bioengineering. 100 (2): 371–378. doi:10.1002/bit.21766. PMID 18175359.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b Hiroaki Okazaki, Fumiko Tazoe, Sachiko Okazaki, Naoyuki Isoo, Kazuhisa Tsukamoto, Motohiro Sekiya, Naoya Yahagi, Yoko Iizuka, Ken Ohashi, Tetsuya Kitamine, Ryu-Ichi Tozawa, Toshihiro Inaba, Hiroaki Yagyu, Mitsuyo Okazaki, Hitoshi Shimano, Norihito Shibata, Hiroyuki Arai, Ryo-Zo Nagai, Takashi Kadowaki, Jun-Ichi Osuga & Shun Ishibashi (September 2006). "Increased cholesterol biosynthesis and hypercholesterolemia in mice overexpressing squalene synthase in the liver". Journal of Lipid Research. 47 (9): 1950–1958. doi:10.1194/jlr.M600224-JLR200. PMID 16741291.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Davidson, MH (January 2007). "Squalene synthase inhibition: a novel target for the management of dyslipidemia". Curr Atheroscler Rep. 9 (1): 78–80. doi:10.1007/BF02693932. PMID 17169251.
{{cite journal}}: CS1 maint: date and year (link) - ^ H. Hiyoshi, M. Yanagimachi, M. Ito, T. Saeki, I. Yoshida, T. Okada, H. Ikuta, D. Shinmyo, K. Tanaka, N. Kurusu & H. Tanaka (November 2001). "Squalene synthase inhibitors reduce plasma triglyceride through a low-density lipoprotein receptor-independent mechanism". European Journal of Pharmacology. 431 (3): 345–352. doi:10.1016/s0014-2999(01)01450-9. PMID 11730728.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Stephanie Seiki & William H. Frishman (March–April 2009). "Pharmacologic inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway: a new therapeutic approach to treatment of hypercholesterolemia". Cardiology in Review. 17 (2): 70–76. doi:10.1097/CRD.0b013e3181885905. PMID 19367148.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: date format (link) - ^ Charlton-Menys V, Durrington PN (2007). "Squalene synthase inhibitors : clinical pharmacology and cholesterol-lowering potential". Drugs. 67 (1): 11–6. doi:10.2165/00003495-200767010-00002. PMID 17209661.
- ^ "RPR 107393, a Potent Squalene Synthase Inhibitor and Orally Effective Cholesterol-Lowering Agent: Comparison with Inhibitors of HMG-CoA Reductase". 1997.
- ^ Do R, Kiss RS, Gaudet D, Engert JC (January 2009). "Squalene synthase: a critical enzyme in the cholesterol biosynthesis pathway". Clin. Genet. 75 (1): 19–29. doi:10.1111/j.1399-0004.2008.01099.x. PMID 19054015.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Liu, CI; et al. (2008). "A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus aureus Virulence". Science. 319 (5868): 1391–1394. doi:10.1126/science.1153018. PMC 2747771. PMID 18276850.
External links[edit]
- Farnesyl-Diphosphate+Farnesyltransferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)


