User:Project Osprey/sandbox4
Consider scoping a production plant to produce vehicle fuels on the Moon in 2055 and make a recommendation as to whether to invest. State all assumptions and researched information (with references, hyperlinks are fine) and show all calculations and working. Comment on how you would progress to make the proposal more certain if you were to go ahead. This is intended to test your ability to think through a problem from a high-level perspective, to make sensible assumptions and calculations and to link a problem to a business case. Communication will also be assessed, but not how the information is presented visually or for spelling and grammar. Feel free to make use of any resources (including help from others) which would help you with this assessment, there are no tools which we consider to be ‘cheating’. We would expect you to spend around 2 hours on this and provide a high-level summary only- there is no strict time limit but please take this into account
To apply for this role, I am also doing things a little differently. Technical reports and executive summaries are wonderfully informative but rarely a joy to read. Every candidate wants a prospective employer to enjoy reading their application and as you've opted for an unconventional application process, I'm going to respond with an unconventional narrative writing style.
The production of vehicle fuels on the Moon would likely be completely unnecessary for Luna vehicles such as rovers or buggies which would be better provided for by a mix of solar and battery technology. Luna fuel production supporting a satellite refuelling programme could become viable investment, however it would be premature to invest now as their remains significant uncertainty about when or even if a Luna base will be established.
When considering the production if vehicle fuels on the moon traditional fuels can be disregarded immediately for a whole range of reasons, an obvious start being the lack of air to support their combustion. Alternative fuels include radioisotope thermoelectric generators (RTG), hydrogen fuel-cells or batteries. RFGs have been used in space since the 1960s[1] and were left on the moon in 1969 by the Apollo 12 crew. Both the UK[2] as well as Russian and China[3] have announced plans to build new compact reactors that can be launched to the moon to support future bases - but the prospect of building nuclear fuel production on the moon itself is a very different. For a start uranium is of low abundance[4] and the technology to mine it in space does not currently exist.[5]
By contrast, hydrogen and oxygen for use in fuel cells can be produced fairly easily by electrolysis of water, which may be shipped to the moon or extracted from the surface ices believed to exist in southern craters.[6] The question here is why bother? At the Moon's South Pole, where bases will be likely located, some locations get sunlight 80% of the time allowing for abundant use of solar energy. Rather than using electricity to split water it would be simpler to just store the electricity for later use. The terrestrial switch-over to electric vehicles is leading to rapid development in battery technology and by 2055 it is likely that any lunar vehicle would be all electric. Large scale battery storage would also serve as a back-up power source for any fixed lunar base, this raises the possibility of a single fully-charged cell being physically transferred to a vehicle to avoid prolonged charging times - literally changing the batteries. However hydrogen and oxygen made from luna ice may have use as vehicle fuels off-world.
Around 7,560 active satellites currently orbit the earth,[7] and their lifespan is limited by their ability to perform manoeuvres which maintain and correct their orbits (orbital station-keeping). In short, satellites contain fuel and once this is exhausted they become unusable. Satellite refuelling has been proposed by several companies and Northrop Grumman successfully demoed the procedure in 2020,[8] however it has yet to be fully commercialised due in-part to the high costs involved. The reason for considering the use of fuel produced on the Moon is that the energy needed to launch material into low earth orbit is only ~5% of that needed to launch the same mass from the earth. Luna escape velocity is sufficiently low that launch could be achieved via electromagnetic catapult using existing technology.[9]. Additionally, the refuelling probes could themselves be reusable, as soft landing on the moon can be achieved fairly easily and without the need of a complex re-entry system. A reusable refuelling feel launched without the high costs of rocketry could make lunar derived fuel economical to use, even if its production cost was considerably higher than equivalent production on Earth. Orbital station-keeping is most commonly achieved with ion thrusters using xenon as a propellant, however the use of oxygen/hydrogen blends has been demonstrated.[10] The provision of a satellite refuelling service is one of the few obvious ways in which a permanent moon base could generate a commercial income, at least in the near term. As such, government assistance in deploying the necessary technology can be regarded as likely. Despite this investing now would doubtless be premature. Plans for a future moon base could easy change or be cancelled making the fuel production plant difficult to scope out with any confidence.
Perhaps you're looking for candidates to display critical thinking skills and demonstrate that they will push back against bad ideas. If so, fair enough, for what it's worth I think
| Nuclear magnetic resonance (NMR) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core concepts | |||||||||||||||
|
|||||||||||||||
| By isotope | |||||||||||||||
|
Trixylylphosphate[edit]

| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| EC Number |
|
| Properties | |
| C24H27O4P | |
| Molar mass | 410.450 g·mol−1 |
| Appearance | Viscous liquid |
| Density | 1.142 |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 394 °C (741 °F; 667 K) |
| 20 µg/L | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H360F | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trixylyl phosphate (TXP) is an aromatic phosphate ester historically used as a hydraulic fluid.
Chirality[edit]
| Chemical Chirality | ||||
|---|---|---|---|---|
| An aspect of Stereoisomerism | ||||
| Core concepts | ||||
|
||||
| Assigning and naming | ||||
|
| Rotational axis (Cn) |
Improper rotational elements (Sn) | ||
|---|---|---|---|
| Chiral no Sn |
Achiral mirror plane S1 = σ |
Achiral inversion centre S2 = i | |
| C1 | 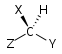 |
 |

|
| C2 |  |
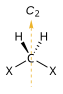 |

|
Uric[edit]
 |
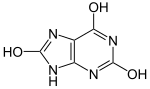
| |
| Lactam form | Lactim form |
 |
 |
pKa1 |
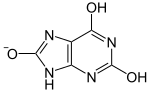
| |
| Lactam form | Lactim form | Urate ion |
Thermosets example table[edit]
| Name | Properties | Applications |
|---|---|---|
| Example | Example | Example |
| Example | Example | Example |
| Example | Example | Example |
formaldehyde base
- Bakelite, a phenol-formaldehyde
- Urea-formaldehyde
- Melamine resin
- Benzoxazines, formed by the reaction of phenols, formaldehyde and primary amines
- Duroplast
- Diallyl-phthalate (DAP)
- Epoxy resin
- Epoxy novolac resins
- Polyimides and Bismaleimides
- Cyanate esters or polycyanurates
- Furan resins
- Silicone resins
- Thiolyte,
- Vinyl ester resins used for wet lay-up laminating, molding and fast setting industrial protection and repair materials.
- Polyester resin fiberglass systems: sheet molding compounds and bulk molding compounds; filament winding; wet lay-up lamination; repair compounds and protective coatings.
- Polyurethanes: insulating foams, mattresses, coatings, adhesives, car parts, print rollers, shoe soles, flooring, synthetic fibers, etc. Polyurethane polymers are formed by combining two bi- or higher functional monomers/oligomers.
- Polyurea/polyurethane hybrids used for abrasion resistant waterproofing coatings.
- Vulcanized rubber.
- Bakelite, a phenol-formaldehyde resin used in electrical insulators and plasticware.
- Duroplast, light but strong material, similar to Bakelite used for making car parts.
- Urea-formaldehyde foam used in plywood, particleboard and medium-density fiberboard.
- Melamine resin used on worktop surfaces.[11]
- Diallyl-phthalate (DAP) used in high temperature and mil-spec electrical connectors and other components. Usually glass filled.
- Epoxy resin [12] used as the matrix component in many fiber reinforced plastics such as glass-reinforced plastic and graphite-reinforced plastic; casting; electronics encapsulation; construction; protective coatings; adhesives; sealing and joining.
- Epoxy novolac resins used for printed circuit boards, electrical encapsulation, adhesives and coatings for metal.
- Benzoxazines, used alone or hybridised with epoxy and phenolic resins, for structural prepregs, liquid molding and film adhesives for composite construction, bonding and repair.
- Polyimides and Bismaleimides used in printed circuit boards and in body parts of modern aircraft, aerospace composite structures, as a coating material and for glass reinforced pipes.
- Cyanate esters or polycyanurates for electronics applications with need for dielectric properties and high glass temperature requirements in aerospace structural composite components.
- Mold or mold runners (the black plastic part in integrated circuits or semiconductors).
- Furan resins used in the manufacture of sustainable biocomposite construction,[13] cements, adhesives, coatings and casting/foundry resins.
- Silicone resins used for thermoset polymer matrix composites and as ceramic matrix composite precursors.
- Thiolyte, an electrical insulating thermoset phenolic laminate material.
- Vinyl ester resins used for wet lay-up laminating, molding and fast setting industrial protection and repair materials.
table[edit]
|
- ^ Schwartz, H. J.; Shure, L. I. (1 January 1965). "Survey of electric power plants for space applications". NTRS - NASA Technical Reports Server. Retrieved 25 April 2024.
- ^ "Government signs £2.9m Moon base nuclear power deal with Rolls-Royce". BBC News. 17 March 2023.
- ^ Baker, Harry (12 March 2024). "Russia and China announce plan to build shared nuclear reactor on the moon by 2035, 'without humans'". Space.com.
- ^ Yamashita, N.; Hasebe, N.; Reedy, R. C.; Kobayashi, S.; Karouji, Y.; Hareyama, M.; Shibamura, E.; Kobayashi, M.‐N.; Okudaira, O.; d'Uston, C.; Gasnault, O.; Forni, O.; Kim, K. J. (May 2010). "Uranium on the Moon: Global distribution and U/Th ratio". Geophysical Research Letters. 37 (10). doi:10.1029/2010GL043061.
- ^ Hedrick, Gabrielle (4 March 2023). "Towards Mining Rare Earth Elements on the Moon". 2023 IEEE Aerospace Conference: 1–11. doi:10.1109/AERO55745.2023.10116027.
- ^ Rubanenko, Lior; Venkatraman, Jaahnavee; Paige, David A. (August 2019). "Thick ice deposits in shallow simple craters on the Moon and Mercury". Nature Geoscience. 12 (8): 597–601. doi:10.1038/s41561-019-0405-8.
- ^ "Satellite Database: Union of Concerned Scientists". www.ucsusa.org.
- ^ "Two commercial satellites link up in space for first time – Spaceflight Now". Spaceflight Now Inc.
- ^ McNab, Ian R.; McGlasson, Benjamin T. (October 2022). "Lunar Electromagnetic Mass Accelerator (LEMMA): An Initial Concept Assessment". IEEE Transactions on Plasma Science. 50 (10): 3326–3333. doi:10.1109/TPS.2022.3176218.
- ^ Tejeda, J.M.; Potrivitu, G.-C.; Azevedo, E. Rosati; Moloney, R.; Knoll, A. (June 2024). "Experimental demonstration of a water electrolysis Hall Effect Thruster (WET-HET) operating with a hydrogen cathode". Acta Astronautica. 219: 542–554. doi:10.1016/j.actaastro.2024.03.043.
- ^ Roberto C. Dante, Diego A. Santamaría and Jesús Martín Gil (2009). "Crosslinking and thermal stability of thermosets based on novolak and melamine". Journal of Applied Polymer Science. 114 (6): 4059–4065. doi:10.1002/app.31114.
- ^ Enrique Guzman; Joël Cugnoni; Thomas Gmür (2014). "Multi-factorial models of a carbon fibre/epoxy composite subjected to accelerated environmental ageing". Composite Structures. 111 (4): 179–192. doi:10.1016/j.compstruct.2013.12.028.
- ^ T Malaba, J Wang, Journal of Composites, vol. 2015, Article ID 707151, 8 pages, 2015. doi:10.1155/2015/707151
- ^ Cite error: The named reference
PP&Awas invoked but never defined (see the help page). - ^ Cite error: The named reference
Geyer2017was invoked but never defined (see the help page).
