User:Posner.c/sandbox
Gyrification[edit]
From Wikipedia, the free encyclopedia

Gyrification is the process of forming the characteristic folds of the cerebral cortex.[1] The peak of such a fold is called a gyrus(plural: gyri), and its trough is called a sulcus (plural: sulci). In most mammals, gyrification usually begins during embryogenesis and fetal development when neurogenesis builds the neuronal cortical layers. Primates, cetaceans, and ungulates have extensive cortical gyri, with a few species exceptions. Gyrification in these animals continues well into postnatal life. The buildup of folds in the brain results in a larger cortical surface area. This is known to increase the speed of brain cell communication, because cortical folds allow for cells in the same pathways to be closer to each other, which in turn requires less energy to send those signals shorter distances.[1] There is also evidence to suggest a positive relationship between brain gyrification and cognitive function processes[2] such as faster reaction times and information processing speed, and better verbal working memory.[3]
While gyrification allows for a larger cortical surface area in a small cranium, the external restraint of the skull is not what causes the folding to occur. Studies have shown that cortical folding can still occur without external constraints.[4] Furthermore, skull growth may actually be driven by brain growth, suggesting that mechanical and genetic factors together may give a better explanation to cortical folding than external constraints.[5] The only observed role that the skull plays in gyrification is in flattening gyri. [4]
Context in Human Brain Development[edit]
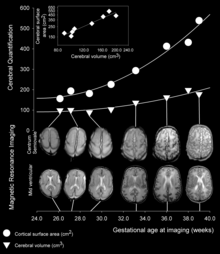
In the human brain, cortical folding takes place during 24 and 32 weeks of gestation.[6] As development proceeds, gyri and sulci begin to take shape with the emergence of deepening indentations on the surface of the cortex. Not all gyri begin to develop at the same time. Instead, the primary cortical gyri form first (beginning as early as gestational week 10 in humans), followed by secondary and tertiary gyri later in development.[3] One of the first and most prominent sulci is the lateral sulcus (also known as the lateral fissure or Sylvian fissure).
Theories[edit]
The mechanisms of cortical gyrification are not well understood, and several hypotheses are debated in the scientific literature. A popular hypothesis dating back to the time of Retzius in the late 19th century asserts that mechanical buckling forces due to the expanding brain tissue cause the cortical surface to fold.(source 4) Many theories since have been loosely tied to this hypothesis.
Theory of Axonal Tension[edit]
An alternative theory, involving axonal tension forces has also been heavily cited.[5] Axonal tension suggests that the tension caused by axons of migrating cortical neurons is what induces folds at specific brain regions. Therefore, in any given gyri , there should be a greater number of connected axons than in two or more different gyri.
There is some evidence to support this hypothesis. For example, axons in areas of the brain with high gyrification levels are straight, short in length, and found to connect gyral walls. This evidence, however, cannot be determined to be a cause or result of cortical folding. While short connections may be a result of axons pulling on the walls of forming gyri, the axons may adaptively remain short due to mechanical growth patterns.[5]
Theory of Differential Tangential Expansion[edit]
More recently, the theory of differential tangential expansion has been investigated in related research, which states that folding patterns of the brain are formed by inconsistent growth rates between the outer cortical layer (grey matter) and the subcortical core (white matter). Though this difference in growth rate produces patterns of gyri and sulci that are consistent with those observed in a natural brain, there is still debate over whether the patterns are driven by isotropic growth or tangential growth, or a combination of the two.
Studies that have modeled this hypothesis with the most success propose that in early neural development, the cortex functionally acts as an outer shell, bonded to a core. Inconsistent growth rates between the two layers cause the outer shell to buckle.[5]
Mechanical Factors[edit]
Cortical Thickness[edit]
Early conditions of the brain have a strong influence on its final level of gyrification. In particular, there is an inverse relationship between cortical thickness and gyrification. Areas of the brain with low values of thickness are found to have higher levels of gyrification. The reverse is also true, that areas of the brain with high values of thickness are found to have lower levels of gyrification.[1]
Growth Speed[edit]
There is some dispute over the growth rates through which cortical and subcortical layers of the brain develop. Purely isotropic growth suggests that the grey (outer shell) and white matter (inner core) layers each grow at separate rates, that are uniform in all dimensions. Tangential growth suggests that the grey matter grows at a faster rate than the inner white matter, and that the growth rate of the grey matter determines the growth rate of the white matter. Though both methods are differential, with the cortex growing more rapidly than the subcortex, tangential growth has been suggested as a more plausible model.[1]
Creases on the brain's surface are formed as a result of instability, and tangential growth models reach levels of instability that cause creasing more frequently than isotropic models. This level is called a critical point, at which, the models prefer to release potential energy by destabilizing and forming creases to become more stable.[5]
Genetic Factors[edit]
The pattern of cortical gyri and sulci is not random; most of the major convolutions are conserved between individuals and many are also found across species. This reproducibility may suggest that genetic mechanisms can specify the location of major gyri. Studies of monozygotic and dizygotic twins of the late 1990's support this idea,[9] as well as later studies suggesting that there may be little heritability in gyrification but the primary gyri and sulci.[7] Comparing surface morphologies of twins show varied secondary and tertiary folds and more highly conserved primary gyri and sulci. Therefore, the secondary and tertiary folds must be more greatly influenced by environmental and physical factors.[8]
Cortical neurogenesis is a cellular process that leads to the instability which causes gyrification. Cortical stem cells, known as radial glial cells (RGC)s, reside in the ventricular zone and generate the excitatory glutamatergic neurons of the cerebral cortex.(sources 15 and 16) These cells rapidly proliferate through self-renewal at early developmental stages, expanding the progenitor pool and increasing cortical surface area. Cortical neurogenesis begins to deplete this pool of progenitor cells, subject to the influences of genetic and environmental cues such as fibroblast growth factors (FGF)s and Notch proteins.(source 17) RGCs generate intermediate neuronal precursors that divide further in the subventricular zone (SVZ), amplifying the number of cortical neurons being produced.(source 18) A second class of RGC, termed basal RGCs (bRGC)s, forms a third progenitor pool in the outer SVZ.(source 19) Basal RGCs are generally much more abundant in higher mammals. Scientific literature points to differences in the dynamics of proliferation and neuronal differentiation in each of these progenitor zones across mammalian species, and such differences may account for the large differences in cortical size and gyrification among mammals. One hypothesis suggests that certain progenitor cells generate abundant neurons destined for the outer cortical layers, causing greater surface area increase in the outer layers compared with the inner cortical layers.(source 6) It remains unclear how this may work without further mechanistic elements.(source 7 and 8)
Regarding particular genes, the DNA-binding factor Trnp1 has been shown to control the expression of genes that play roles in cortical expansion, as well as regulating the reproduction of neural progenitor cells and radial glial cells.[5] The migration of these neural stem cells to the outer layers of the brain may be the driving force behind gyrification.
Variation Across Species[edit]

Gyrification varies greatly across different speices. In general, more lissencephalic (smooth) brains are found in smaller species, and gyrencephalic brains found in larger ones.[2]
Among small animals with lissencephalic brains are those without ridges altogether, such as the mouse, which normally does not develop cortical convolutions. Studies have attempted to induce cortical gyrification in the mouse with varying degrees of success. (sources 10 and 11 in original) The first viable mouse model of gyrencephaly was reported in 2013, when fully layered, organized neocortical gyri were induced to form in the mouse brain. (source 12). Furthermore, induction of gyrus formation occurred before axons could play a role, arguing against the axonal tension hypothesis during primary gyrification. Nevertheless, in primates many tertiary gyri do not form until well after the last cortical neurons have been generated, and it is likely that a number of factors, including axonal innervation, contribute to the maturation of cortical gyri.(sources 13 and 14). These studies together showed that changes in stem cell dynamics can have profound effects on brain structure, and provide potential clues to the evolution of gyrencephaly. (source??)
Some mammals, such as the ferret, are born with smooth brains,[9] and develop gyri mainly after birth.
Roles in Neurological Disorders[edit]
Lissencephaly[edit]
A cerebral cortex lacking surface convolutions is said to be lissencephalic, meaning 'smooth-brained'.(source 2) During embryonic development, all mammalian brains begin as lissencephalic structures derived from the neural tube. Some, like mice brains, remain lissencephalic throughout adulthood.
For humans with lissencephaly, a large portion of neurons fail to reach the outer cortex during neuronal migration, and remain under the cortical plate.[6] This displacement results in not only defects in cortical connections, but also a thickened cortex, consistent with the idea that a brain with a thicker cortex will have lower levels of gyrification.[10]
Polymicrogyria[edit]
Polymicrogyria is a condition in which the brain has an overly convoluted cortex. Though at the surface, the brain appears smooth with a few sulci, looking at the interior of the brain reveals a convoluted structure with a large number of secondary and tertiary folds.[10] Brain imaging with MRI reveals a brain with polymicrogyria to have a thin cortex, consistent with the idea that a brain with a thin cortex will have a high level of gyrification.[6][10]
Autism[edit]
Patients with autism have overall higher levels of cortical gyrification,[11] but only in the temporal, parietal, and occipital lobes, as well as part of the cingulate cortex.[12] The higher levels of gyrification are found to relate to greater local connectivity in autistic brains, suggesting hyperconnectivity.
The folds of autistic human brains are found to experience slight shifts in location, early brain development. Specifically, different patterns appear in the superior frontal sulcus, Sylvian fissure, inferior frontal gyrus, superior temporal gyrus, and olfactory sulci.[13] These areas relate to working memory, emotional processing, language, and eye gaze,[14] and their difference in location and level of gyrification when compared to a healthy human brain could explain some altered behaviors in autistic patients.
Schizophrenia[edit]
A more prevalent condition, schizophrenia, has been associated with structural abnormalities in the brain as well. Like autistic brains, schizophrenic brains show reduced cortical thickness and increased gyrification when compared to healthy brains.[6][15]
References[edit]
Additional images[edit source | edit][edit]
- Various brains. Clockwise from top left: Adult rhesus; Adult mouse; Midgestation human; Newborn human; Adult human.
- Normal human adult cerebrum (left),polymicrogyria (center) and lissencephaly (right).
 | This is a user sandbox of Posner.c. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
- ^ a b c Striedter, Georg F.; Srinivasan, Shyam; Monuki, Edwin S. (2015-01-01). "Cortical Folding: When, Where, How, and Why?". Annual Review of Neuroscience. 38 (1): 291–307. doi:10.1146/annurev-neuro-071714-034128. PMID 25897870.
- ^ a b Sun, Tao; Hevner, Robert F. "Growth and folding of the mammalian cerebral cortex: from molecules to malformations". Nature Reviews Neuroscience. 15 (4): 217–232. doi:10.1038/nrn3707. PMC 4107216. PMID 24646670.
- ^ Gautam, Prapti; Anstey, Kaarin J.; Wen, Wei; Sachdev, Perminder S.; Cherbuin, Nicolas (2015-07-01). "Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults". Behavioural Brain Research. 287: 331–339. doi:10.1016/j.bbr.2015.03.018.
- ^ a b Tallinen, Tuomas; Chung, Jun Young; Biggins, John S.; Mahadevan, L. (2014-09-02). "Gyrification from constrained cortical expansion". Proceedings of the National Academy of Sciences. 111 (35): 12667–12672. doi:10.1073/pnas.1406015111. ISSN 0027-8424. PMC 4156754. PMID 25136099.
- ^ a b c d e Striedter, Georg F.; Srinivasan, Shyam; Monuki, Edwin S. "Cortical Folding: When, Where, How, and Why?". Annual Review of Neuroscience. 38 (1): 291–307. doi:10.1146/annurev-neuro-071714-034128.
- ^ a b c d Budday, Silvia; Raybaud, Charles; Kuhl, Ellen (2014-07-10). "A mechanical model predicts morphological abnormalities in the developing human brain". Scientific Reports. 4. doi:10.1038/srep05644. PMC 4090617. PMID 25008163.
- ^ White, Tonya; Su, Shu; Schmidt, Marcus; Kao, Chiu-Yen; Sapiro, Guillermo (2010-02-01). "The Development of Gyrification in Childhood and Adolescence". Brain and cognition. 72 (1): 36. doi:10.1016/j.bandc.2009.10.009. ISSN 0278-2626. PMC 2815169. PMID 19942335.
- ^ Gómez-Robles, Aida; Hopkins, William D.; Sherwood, Chet C. (2013-06-22). "Increased morphological asymmetry, evolvability and plasticity in human brain evolution". Proc. R. Soc. B. 280 (1761): 20130575. doi:10.1098/rspb.2013.0575. ISSN 0962-8452. PMC 3652445. PMID 23615289.
- ^ Neal, Jason; Takahashi, Masaya; Silva, Matthew; Tiao, Grace; Walsh, Christopher A; Sheen, Volney L (2007-01-01). "Insights into the gyrification of developing ferret brain by magnetic resonance imaging". Journal of Anatomy. 210 (1): 66–77. doi:10.1111/j.1469-7580.2006.00674.x. ISSN 0021-8782. PMC 2100265. PMID 17229284.
- ^ a b c Ross, M Elizabeth; Walsh, Christopher A. "H UMAN B RAIN M ALFORMATIONS AND T HEIR L ESSONS FOR N EURONAL M IGRATION". Annual Review of Neuroscience. 24 (1): 1041–1070. doi:10.1146/annurev.neuro.24.1.1041.
- ^ Wallace, Gregory L.; Robustelli, Briana; Dankner, Nathan; Kenworthy, Lauren; Giedd, Jay N.; Martin, Alex (2013-06-01). "Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders". Brain. 136 (6): 1956–1967. doi:10.1093/brain/awt106. ISSN 0006-8950. PMC 3673467. PMID 23715094.
- ^ Yang, Daniel Y.-J.; Beam, Danielle; Pelphrey, Kevin A.; Abdullahi, Sebiha; Jou, Roger J. (2016-01-25). "Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification". Molecular Autism. 7 (1). doi:10.1186/s13229-016-0076-x. PMC 4727390. PMID 26816612.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chen, Jason A.; Peñagarikano, Olga; Belgard, T. Grant; Swarup, Vivek; Geschwind, Daniel H. "The Emerging Picture of Autism Spectrum Disorder: Genetics and Pathology". Annual Review of Pathology: Mechanisms of Disease. 10 (1): 111–144. doi:10.1146/annurev-pathol-012414-040405.
- ^ Levitt, Jennifer G.; Blanton, Rebecca E.; Smalley, Susan; Thompson, P. M.; Guthrie, Donald; McCracken, James T.; Sadoun, Tania; Heinichen, Laura; Toga, Arthur W. (2003-07-01). "Cortical Sulcal Maps in Autism". Cerebral Cortex. 13 (7): 728–735. doi:10.1093/cercor/13.7.728. ISSN 1047-3211. PMID 12816888.
- ^ http://www.sciencedirect.com/science/article/pii/S000632231001303X
