User:Ninja Recs/sandbox
Article Evaluation
Assignment Topic[edit]
== IRON-SULFUR CLUSTERS ARTICLE[edit]
-The article presents a simple definition for iron-sulfur cluster with very limited info and explanation of their examples. More information of the diagram and knowledge can be applied.
-The current information stated in the article can be updated. In addition, electron counting can be displayed to show the contributions of the ligands to the metal. Also define the strength of their bonds, " hard soft".
-More sources and studies are required. This article is also stated as Mid-Improtance in the Stub Class.
Assignment Topics ===[edit]
From the article Iron-Sulfur clusters, many subtopics can be applied. However, I will be focusing on the following:
- Briefly explaining what are Iron-Sulfur cluster and providing more examples.
- Adding more information regarding the strength of the Iron and Sulfur itself, and showing the electron counting for those diagrams present.
- Applications of Iron-sulfur cluster and their purpose
- Lastly their electron transfer effects and covalency.
Sources I will be Using are the following: [1]
First 250 words Contribution[edit]
In the iron-sulfur cluster it is important to understand the strength of the bonds between them. Sulfur is a soft metal ion, due to its electromagnetically. Where as Iron it becomes complex, since it is also an Soft metal but it can also be hard. This make Iron to be an intermediate metal, it can bind to both hard and soft. Since, Sulfur is soft, we assume the Iron metal bonded to it is also soft. But we do not know its strength. Additionally, the clusters of these iron-sulfur bond is more complex, since there are more than 1 iron-sulfur bonds presented in the Article. Ligand contributions and Electron counting would provide more information as well as their covalency of their bonds. [2] For example, in an previous experiment for measuring bond strength the researchers found that the bond between Fe(II)–S(Met) was equal to 2.6 (kcal/ mol), where as the Fe(III)–S(Met) bond was much stronger; 5.9 (kcal/mol). This is important, since initially the Fe(II)-S(Met) bond was theorized to have the stronger bond due to short bond lengths. However, after analysis through the X-Ray Absorption they found that it wasn't the case, hence those values mentioned resulted.[3]
In addition, molecular orbital diagrams of both Iron(II) and Iron(III) with other ligands can provide further information on the high-spin and low-spin states. This information can provide the electron repulsion and attraction between the bonds. Since, there are many bonds of Iron and Sulfur due to its cluster, we have to consider the bridging effects, which is where covalency will play its part. Does this bridging connection have any effects on their redox or energy potential[4]. The covalent bond between Iron & Sulfur is vital, since it provides information on its stability. The lone pairs of the Sulfur are tricky and can react with other metals. However, due to the bridging bonds of more than one Iron present this can limit the reactivity of Sulfur towards other metals.[5]
900 WORD CONTRIBUTION[edit]
The human body consist of millions and millions of cells, each requiring energy for many specific functions to be performed. ATP synthase is one common way that our bodies produce energies from. However, it requires help from other membranes and also proteins. Ferredoxin is a protein in particular which aids in the transfer of electrons through Iron-Sulfur Clusters. These clusters are very complex and are critical when the reaction is taking place. Firstly, there are many types of cluster such as the following: [2Fe-2S], [4Fe-3S], [3Fe-4S] and lastly our primary focus will be the [4Fe-4S] complex[6]. What the coefficient or numbers represent in front of the metal represents how many metals there are. For example, for the [2Fe-2S] complex it has a linear rhombic shape. On the other hand, the [4Fe-4S] complex has a cubic shape with all metals binding to one another. A figure of both complexes are shown below:
The main difference between these complexes apart from their structure is that the [4Fe-S] cluster has more high potential ferredoxins. This means that they have more variation of charges that the complex can consist of. Rather than the simple cluster of [2Fe-2S] only having 3 variations such as Fe (II) – Fe (II) or Fe (III) – Fe (III) etc.: The cubic cluster consists of more outcomes (12 to be exact) such as Fe(III) – Fe(II) – Fe(II) - Fe(III) etc.: These clusters are very important, luckily we have an abundant source of them. These clusters can be found in many bacteria, archaea and some eukaryotes[7]. Secondly, the interaction of Fe and S is very vital. This is because the bond between an Fe (III) – S is very different from an Fe (II) – S bond. A cluster with higher net charges with 2 Fe (III) would be harder to reduce than the cluster with a lower net charge.[8] This phenomenon is due to the energy required to break the bond and in addition, the Fe (III) cluster maybe more stable. This can be further addressed by applying the Pauli exclusion principle, which states no spin states can be within the same configuration as the other. The Fe (III) – Fe (III) bond, would create a total anti-ferromagnetic coupling equaling to zero. Where as the Fe (III) – Fe (II) would be just an anti-ferromagnetic coupling not equaling to zero. This suggest that this complex is a polar covalent bond due to the electronegativity difference being more than 0 but lower than 2 (approximately ~ 0.8). Also inconsideration, the Fe (III) / (II) is a hard and intermediate metal. This becomes complicated when the bond is with sulfur, since it is restricted and is only a soft metal. The differences in length of bonds can also impact the results of the transfer electrons. For example, in an previous experiment for measuring bond strength the researchers found that the bond between Fe(II)–S(Met) was equal to 2.6 (kcal/ mol), where as the Fe(III)–S(Met) bond was much stronger; 5.9 (kcal/mol). This is important, since initially the Fe(II)-S(Met) bond was theorized to have the stronger bond due to short bond lengths. However, after analysis through the X-Ray Absorption they found that it wasn't the case, hence those values mentioned resulted.[3] Another important factor to consider is the covalency of Fe and S. This impacts the shape and structure of the clusters. A high Fe-S covalency will cause reorganization energy due to higher charge. Example, would be the Metal - S (II) charge is very high than an R – S (I). However, through Solomon’s work, from pre-edge XAS studies, he found that H- bonds lowers the covalency hence it compensates for the high covalency initially. Also resonance affects the rigid structures and stabilization of the clusters. Lastly, taking a look at [4Fe-4S] cluster in particular, their electronic structure provides valuable information. As mentioned before this has to do with the anti-ferromagnetic chargers. The [4Fe-4S] with a net charge of 3+ being the most charge would be considering to be the most oxidized. However, through analysis this does not occur since it is completely unstable. However, a [4Fe-4S] with a net charge of +1 would favoured due to its polarity but would still consist of lower stability due to only 1 Fe (III) – Fe (II) bond. this means that the [4Fe-4S] cluster with a charge of 2+ is the most stable, not be default but due to having more mixed pairs of Fe (III) – Fe (II) bonds[9]. Despite the [4Fe-4S] clusters having its benefits and flaws, perhaps the most important flaw to note is the exposure of the clusters and how it can be broken down. As mentioned we understand that [4Fe-4S] with 2+ is most stable however, when this cluster is exposed to Oxygen (O2), it will target and reduce one of Fe (II) bonds and break that metal bond from the complex turning it to a [3Fe-4S] cluster which is not that stable. This is vital, since in the Krebs Cycle / CAC (citric acid cycle), oxygen is present and these clusters can and will react with them. This causes damage and can cause mutations. The Fe bond usually forms an keytone bond when the per-oxo oxygen bond is broken. This is later reduced by a proton and forms a peroxide or alcohol while releasing the Fe (III) bond also. However, the synthesis of ATP is vital and there are phenomena’s which can minimize and prevent these exposures but that’s a study for next time.
Week 3 Tasks - Chromic Acetate[edit]
 α-D-glucopyranose (chair form)
| |
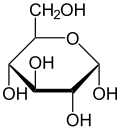 Haworth projection of α-D-glucopyranose
| |
 Fischer projection of D-glucose
| |
| Names | |
|---|---|
| Pronunciation | /ˈɡluːkoʊz/, /ˈɡluːkoʊs/ |
| Preferred IUPAC name
D-Glucose | |
| Systematic IUPAC name
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal | |
| Other names
Blood sugar
Dextrose Corn sugar D-Glucose Grape sugar | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| Abbreviations | Glc |
| 1281604 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 83256 | |
| KEGG | |
| MeSH | Glucose |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Thermochemistry | |
Heat capacity (C)
|
218.6 J K−1 mol−1[10] |
Std molar
entropy (S⦵298) |
209.2 J K−1 mol−1[10] |
Std enthalpy of
formation (ΔfH⦵298) |
−1271 kJ/mol[11] |
| 2,805 kJ/mol (670 kcal/mol) | |
| Pharmacology | |
| B05CX01 (WHO) V04CA02 (WHO), V06DC01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | ICSC 08655 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
CHROMIUM(III)TRIACETATE[edit]
- MOLECULAR WEIGHT - 229.128 g/mol
- MOLECULAR FORMULA - C6H9CrO6
- BOILING POINT - 212 ° F
- MELTING POINT - >400°C
- BLUE-GREENISH POWDER
- DENSITY - 1.56 g/cu cm
- SOLUBILITY - 675 g/L at 20°C
CHROMIUM(III)TRIACETATE
Sigma-Aldrich catalog entry for [12]
| Safety/Hazards | Toxicity |
| Inhalation warning, can cause irritating and can damage respiratory system | Carcinogen Risks |
| Don't expose to skin, can cause dermatitis | Cr(II) and Cr(III) radicals can cause effects |
- ^ SOLOMAN, EDWARD. "LIGAND X-RAY ABS SPEC OF METAL COVALENCY".
{{cite web}}: Missing or empty|url=(help) - ^ SOLOMON, EDWARD. https://pubs.acs.org/doi/abs/10.1021/ic0203320?journalCode=inocaj.
{{cite web}}: Missing or empty|title=(help) - ^ MICHAEL, MARA. http://science.sciencemag.org/content/356/6344/1276.
{{cite web}}: Missing or empty|title=(help) - ^ SOLOMON, EDWARD. http://science.sciencemag.org/content/356/6344/1276.
{{cite web}}: Missing or empty|title=(help) - ^ SOLOMON, EDWARD. https://pubs.acs.org/doi/abs/10.1021/ar990125c.
{{cite web}}: Missing or empty|title=(help) - ^ Lill, Roland. "Issue of iron-sulfur protein". Biochim Biophys Acta. Retrieved 2017.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Fisher, N (1998). "Intramolecular electron transfer in [4Fe-4S)]". The EMBO journal: 849-858.
- ^ Fisher, N (1998). "Intramolecular electron transfer in [4Fe-4S)]". The EMBO journal: 849-858.
- ^ Lagnan, R (1992). "Protein control of iron-sulfur cluster redox potentials". J. BIOL CHEM: 267.
- ^ a b Boerio-Goates, Juliana (1991), "Heat-capacity measurements and thermodynamic functions of crystalline α-D-glucose at temperatures from 10K to 340K", J. Chem. Thermodynam., 23 (5): 403–09, doi:10.1016/S0021-9614(05)80128-4
- ^ Ponomarev, V. V.; Migarskaya, L. B. (1960), "Heats of combustion of some amino-acids", Russ. J. Phys. Chem. (Engl. Transl.), 34: 1182–83
- ^ Pubchem. "Chromic acetate". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-09-27.

