User:Mr. Ibrahem/Zonisamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zonegran, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603008 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sulfonamide[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100%[2] |
| Protein binding | 40%[2] |
| Metabolism | Liver through CYP3A4[2] |
| Elimination half-life | 63 hours in plasma[2] |
| Excretion | Kidney (62%); Faeces (3%)[2] |
| Identifiers | |
| |
| Chemical and physical data | |
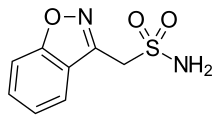
| Formula | C8H8N2O3S |
| Molar mass | 212.22 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 162 °C (324 °F) |
| |
| |
| (verify) | |
Zonisamide, sold under the brand name Zonegran, is a medication used in addition to other medications to treat epilepsy, specifically partial seizures.[1] There is also some evidence for its use in Parkinson's.[3] It is taken by mouth.[1]
Common side effects include abdominal pain, nausea, constipation, dry mouth, and dizziness.[1] Other side effects may include hair loss, nystagmus, and bone marrow disorders.[4] Use during pregnancy may harm the baby and use during breastfeeding is not recommended.[5] It is in the sulfonamide family of chemicals and is not recommended in people allergic to these.[1][4]
Zonisamide was approved for medical use in the United States in 2000.[1] It is available as a generic medication.[6] In the United States it costs less than 20 USD per month as of 2021.[6] It is also relatively inexpensive in the United Kingdom.[4]
References[edit]
- ^ a b c d e f g h "Zonisamide Monograph for Professionals". Drugs.com. Archived from the original on 27 February 2021. Retrieved 4 August 2021.
- ^ a b c d e "Zonegran Product Information" (PDF). TGA eBusiness Services. SciGen (Australia) Pty Ltd. 4 April 2013. Archived from the original on 15 October 2018. Retrieved 18 November 2013.
- ^ Grover ND, Limaye RP, Gokhale DV, Patil TR (November–December 2013). "Zonisamide: a review of the clinical and experimental evidence for its use in Parkinson's disease". Indian Journal of Pharmacology. 45 (6): 547–55. doi:10.4103/0253-7613.121266. PMC 3847242. PMID 24347760.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 350. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Zonisamide (Zonegran) Use During Pregnancy". Drugs.com. Archived from the original on 2 February 2021. Retrieved 5 August 2021.
- ^ a b "Zonisamide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 5 August 2021.
