User:Mr. Ibrahem/Tegaserod
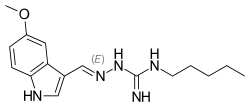 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zelnorm, Zelmac |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10% |
| Protein binding | 98% |
| Metabolism | Stomach and liver |
| Elimination half-life | 11 ± 5 hours |
| Excretion | Fecal and Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C16H23N5O |
| Molar mass | 301.394 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tegaserod, sold under the brand name Zelnorm among others, is a medication used to treat irritable bowel syndrome with constipation.[1] It is taken by mouth.[1]
Common side effects include headache, abdominal pain, diarrhea, and dizziness.[1] Other side effects may include heart attacks, stroke, and suicide.[1] It is a 5-HT4 activator which stimulates both contraction and increased fluid release by the intestines.[1]
Tegaserod was approved for medical use in the United States in 2002.[1] It was removed from the market in 2007 due to concerns related to the heart but than re-allowed in 2019.[1] In Europe it was refused approval in both 2005 and 2006.[2] In the United States it costs about 360 USD per month as of 2021.[3] It was withdrawn from the market due to commercial reasons in 2022.[4]
References[edit]
- ^ a b c d e f g h i j "Tegaserod Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 24 September 2021.
- ^ "Zelnorm". Archived from the original on 6 October 2018. Retrieved 24 September 2021.
- ^ "Zelnorm Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 24 September 2021.
- ^ "Pharmacist's Letter". pharmacist.therapeuticresearch.com. Archived from the original on 8 August 2022. Retrieved 12 January 2023.
