User:Mr. Ibrahem/Stiripentol
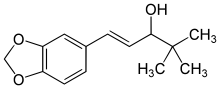 | |
| Clinical data | |
|---|---|
| Pronunciation | stir"i pen' tol |
| Trade names | Diacomit |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618069 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Anticonvulsant[1] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C14H18O3 |
| Molar mass | 234.295 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Stiripentol, sold under the brand name Diacomit, is a medication used to treat a form of epilepsy known as severe myoclonic epilepsy in infancy (SMEI, Dravet syndrome).[4][1] It is taken by mouth.[1] It is taken with clobazam in children over the age of 2 years.[1]
Common side effects include loss of appetite, weight loss, trouble sleeping, sleepiness, and poor coordination.[4] Other side effects may include low neutrophils, low platelets, and suicide.[1] Use during pregnancy may harm the baby.[1] It is a anticonvulsant with the chemical structure of an aromatic allylic alcohol.[1]
Stiripentol was approved for medical use in Europe in 2007 and the United States in 2018.[4][1] In the United Kingdom 250 mg twice per day for a month costs the NHS about £280 as of 2021.[5] In the United States this amount costs about 1,600 USD.[6]
References[edit]
- ^ a b c d e f g h "Stiripentol Monograph for Professionals". Drugs.com. 31 August 2020. Archived from the original on 11 October 2020. Retrieved 8 November 2020.
- ^ "Diacomit 250mg hard capsules - Summary of Product Characteristics (SmPC)". (emc). 31 May 2019. Archived from the original on 13 November 2020. Retrieved 8 November 2020.
- ^ "Diacomit- stiripentol capsule Diacomit- stiripentol powder, for suspension". DailyMed. 15 May 2020. Archived from the original on 6 August 2020. Retrieved 8 November 2020.
- ^ a b c d e f "Diacomit EPAR". European Medicines Agency. Archived from the original on 12 November 2020. Retrieved 8 November 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 347. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Diacomit Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 April 2021. Retrieved 14 October 2021.
