User:Mr. Ibrahem/Smallpox vaccine
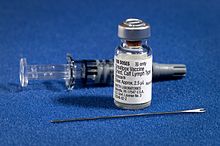 Smallpox vaccine diluent in a syringe alongside a vial of dried Dryvax smallpox vaccine and bifurcated needle. | |
| Vaccine description | |
|---|---|
| Vaccine type | Live virus |
| Clinical data | |
| Trade names | ACAM2000, Imvanex, Jynneos, others |
| Other names | Monkeypox vaccine |
| AHFS/Drugs.com | ACAM2000 Monograph MVA-BN Monograph |
| License data | |
| Pregnancy category | |
| Routes of administration | Subcutaneous |
| Legal status | |
| Legal status | |
Smallpox vaccines are vaccines that prevents smallpox and monkeypox.[3][2] For smallpox it was about 95% effective for 3 to 5 years.[3] For monkeypox it has been at least 85% effective.[4] While it is not routinely given to prevent smallpox, it is available for potential exposures.[5] In 2022, it was recommended for people at high risk of these diseases.[4] Older vaccines are given by scarification while newer ones are given by injection under the skin.[1][2]
Current vaccines include ACAM2000, which was approved for medical use in the United States in 2007 and MVA-BN which was approved in 2019.[1][2] ACAM2000 is a live vaccine that can duplicate itself, while MVA-BN is a live vaccine that cannot duplicate itself.[4] Other types are under investigation.[5]
Common side effects of ACAM2000 include swollen lymph nodes, tiredness, fever, and rash at the site of inoculation.[1] Severe side effects may include myocarditis, eczema vaccinatum, and encephalitis.[1] Use in pregnancy may harm the baby.[1] Common side effects of MVA-BN include pain at the site of injection, tiredness, muscle pain, and headache.[2] Severe side effects may include anaphylaxis.[2] There is no evidence of increased harm in pregnancy or eczema.[2]
Smallpox vaccine was first applied in a scientific manner in 1796, when Edward Jenner demonstrated that infection by the relatively mild cowpox virus conferred protection from smallpox.[6] Cowpox; however, had been used previously in 1774 by Benjamin Jesty and other physicians in the 1700s but had not been promoted to the same degree.[6] The practice of vaccination become common across Europe by 1800.[6] From 1966 to 1977, the World Health Organization conducted a vaccination campaign that eradicated smallpox, making it the only human disease to be eradicated.[6][7] In the 2000s the cost of a replicating vaccine was about 5 USD per dose while that of a non-replicating vaccine was about 29 USD.[8]
References[edit]
- ^ a b c d e f "DailyMed - ACAM2000 (smallpox- vaccinia vaccine, live injection, powder, lyophilized, for solution". dailymed.nlm.nih.gov. Archived from the original on 24 May 2022. Retrieved 26 July 2022.
- ^ a b c d e f g h i "DailyMed - JYNNEOS- vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen injection, suspension". dailymed.nlm.nih.gov. Archived from the original on 27 May 2022. Retrieved 26 July 2022.
- ^ a b c "Vaccine Basics | Smallpox | CDC". www.cdc.gov. 15 February 2019. Archived from the original on 20 May 2022. Retrieved 26 July 2022.
- ^ a b c "Monkeypox and Smallpox Vaccine Guidance | Monkeypox | Poxvirus | CDC". www.cdc.gov. 18 July 2022. Archived from the original on 19 May 2022. Retrieved 26 July 2022.
- ^ a b Bonville, Cynthia; Domachowske, Joseph (2021). "28. Smallpox". In Domachowske, Joseph; Suryadevara, Manika (eds.). Vaccines: A Clinical Overview and Practical Guide. Switzerland: Springer. pp. 337–342. ISBN 978-3-030-58416-0. Archived from the original on 3 June 2022. Retrieved 11 June 2022.
- ^ a b c d Riedel, S (January 2005). "Edward Jenner and the history of smallpox and vaccination". Proceedings (Baylor University. Medical Center). 18 (1): 21–5. doi:10.1080/08998280.2005.11928028. PMID 16200144.
- ^ "Smallpox vaccines". www.who.int. Archived from the original on 13 June 2020. Retrieved 26 July 2022.
- ^ Lambert de Rouvroit, Axel; Heegaard, Erik D. (January 2016). "Total costs associated with replicating and non-replicating smallpox vaccines". Global Security: Health, Science and Policy. 1 (1): 3–9. doi:10.1080/23793406.2016.1171944.
