User:Mr. Ibrahem/Sitagliptin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɪtəˈɡlɪptɪn/ |
| Trade names | Januvia, Tesavel, Xelevia, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 87% |
| Protein binding | 38% |
| Metabolism | Liver (CYP3A4- and CYP2C8-mediated) |
| Elimination half-life | 8 to 14 h[1] |
| Excretion | Kidney (80%)[1] |
| Identifiers | |
| |
| Chemical and physical data | |
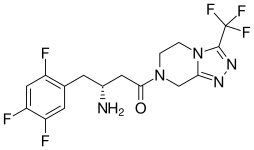
| Formula | C16H15F6N5O |
| Molar mass | 407.320 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Sitagliptin, sold under the brand name Januvia among others, is a medication used to treat diabetes type 2.[2] In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea.[4] It is taken by mouth.[2] It is also available within a single pill as metformin/sitagliptin.[2]
Common side effects include headaches, swelling of the legs, and upper respiratory tract infections.[2] Serious side effects may include angioedema, low blood sugar, kidney problems, pancreatitis, and joint pain.[2] Whether use in pregnancy or breastfeeding is safe is unclear.[5] It is in the dipeptidyl peptidase-4 (DPP-4) inhibitor class and works by increasing the production of insulin and decreasing the production of glucagon by the pancreas.[2]
Sitagliptin was developed by Merck & Co. and approved for medical use in the United States in 2006.[2] A month's supply in the United Kingdom costs the NHS about £33.26 per month as of 2020.[4] In the United States the wholesale cost of this amount is about US$405.00.[6] In 2017, it was the 95th most commonly prescribed medication in the United States, with more than eight million prescriptions.[7][8]
References[edit]
- ^ a b Herman GA, Stevens C, van Dyck K, Bergman A, Yi B, De Smet M, Snyder K, Hilliard D, Tanen M, Tanaka W, Wang AQ, Zeng W, Musson D, Winchell G, Davies MJ, Ramael S, Gottesdiener KM, Wagner JA (December 2005). "Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses". Clin Pharmacol Ther. 78 (6): 675–88. doi:10.1016/j.clpt.2005.09.002. PMID 16338283.
- ^ a b c d e f g h i "Sitagliptin Phosphate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 25 September 2020. Retrieved 6 September 2020.
- ^ a b BNF (80 ed.). London: BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. pp. 734–734. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Sitagliptin Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ^ "Sitagliptin Phosphate - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
