User:Mr. Ibrahem/Nicotinamide
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌnɪkəˈtɪnəmaɪd/ |
| Other names | 3-pyridinecarboxamide niacinamide nicotinic acid amide vitamin PP nicotinic amide vitamin B3 |
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Routes of administration | By mouth, topical |
| Drug class | Vitamin |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Chemical and physical data | |
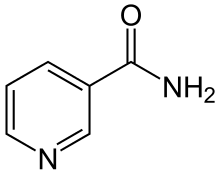
| Formula | C6H6N2O |
| Molar mass | 122.127 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.40 g/cm3[1] g/cm3 |
| Melting point | 129.5 °C (265.1 °F) |
| Boiling point | 334 °C (633 °F) |
| |
| |
Nicotinamide (NAM), also known as niacinamide, is a form of vitamin B3 found in food and used as a dietary supplement and medication.[5][6][7] As a supplement, it is used by mouth to prevent and treat pellagra (niacin deficiency).[6] While nicotinic acid (niacin) may be used for this purpose, nicotinamide has the benefit of not causing skin flushing.[6] As a cream, it is used to treat acne.[7]
Side effects are minimal.[8][9] At high doses liver problems may occur.[8] Normal amounts are safe for use during pregnancy.[3] Nicotinamide is in the vitamin B family of medications, specifically the vitamin B3 complex.[10][11] It is an amide of nicotinic acid.[8] Foods that contain nicotinamide include yeast, meat, milk, and green vegetables.[12]
Nicotinamide was discovered between 1935 and 1937.[13][14] It is on the World Health Organization's List of Essential Medicines.[15] Nicotinamide is available as a generic medication and over the counter.[10] In the United Kingdom a 60 g tube costs the NHS about £7.10.[7] Commercially, nicotinamide is made from either nicotinic acid or nicotinonitrile.[14][16] In a number of countries grains have nicotinamide added to them.[14]
References[edit]
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "NICOTINAMIDE = VITAMIN PP = VITAMIN B3 oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 1 September 2020.
- ^ a b "Niacinamide Use During Pregnancy". Drugs.com. Archived from the original on December 30, 2016. Retrieved December 29, 2016.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 1 September 2020.
- ^ Bender, David A. (2003). Nutritional Biochemistry of the Vitamins. Cambridge University Press. p. 203. ISBN 978-1-139-43773-8. Archived from the original on 2016-12-30.
- ^ a b c World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 496, 500. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c British National Formulary: BNF 69 (69th ed.). British Medical Association. 2015. p. 822. ISBN 978-0-85711-156-2.
- ^ a b c Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA (November 2000). "Safety of high-dose nicotinamide: a review" (PDF). Diabetologia. 43 (11): 1337–45. doi:10.1007/s001250051536. PMID 11126400. S2CID 24763480. Archived (PDF) from the original on 22 September 2017. Retrieved 20 April 2018.
- ^ MacKay D, Hathcock J, Guarneri E (June 2012). "Niacin: chemical forms, bioavailability, and health effects". Nutrition Reviews. 70 (6): 357–66. doi:10.1111/j.1753-4887.2012.00479.x. PMID 22646128.
- ^ a b "Niacinamide: Indications, Side Effects, Warnings". Drugs.com. June 6, 2017. Archived from the original on August 5, 2017. Retrieved June 30, 2017.
- ^ Krutmann, Jean; Humbert, Philippe (2010). Nutrition for Healthy Skin: Strategies for Clinical and Cosmetic Practice. Springer Science & Business Media. p. 153. ISBN 9783642122644. Archived from the original on 2017-04-10.
- ^ Burtis, Carl A.; Ashwood, Edward R.; Bruns, David E. (2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (5th ed.). Elsevier Health Sciences. p. 934. ISBN 978-1-4557-5942-2. Archived from the original on 2016-12-30.
- ^ Sneader, Walter (2005). Drug Discovery: A History. John Wiley & Sons. p. 231. ISBN 978-0-470-01552-0. Archived from the original on 2016-12-30.
- ^ a b c Blum, René (2015). "Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide)". Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide. Ullmann's Encyclopedia of Industrial Chemistry (6th ed.). Weinheim: Wiley-VCH. pp. 1–9. doi:10.1002/14356007.o27_o14.pub2. ISBN 978-3-527-30385-4.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Schmidberger, J. W.; Hepworth, L. J.; Green, A. P.; Flitsch, S. L. (2015). "Enzymatic Synthesis of Amides". In Faber, Kurt; Fessner, Wolf-Dieter; Turner, Nicholas J. (eds.). Biocatalysis in Organic Synthesis 1. Science of Synthesis. Georg Thieme Verlag. pp. 329–372. ISBN 9783131766113. Archived from the original on 2017-11-05.
