User:Mr. Ibrahem/Fulvestrant
 | |
| Clinical data | |
|---|---|
| Pronunciation | /fʊlˈvɛstrənt/ fuul-VES-trənt |
| Trade names | Faslodex, others |
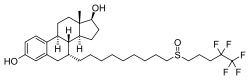
| Other names | ICI-182780; ZD-182780; ZD-9238; 7α-[9-[(4,4,5,5,5-Pentafluoropentyl)-sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection |
| Drug class | Antiestrogen |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low[1] |
| Protein binding | 99%[1] |
| Metabolism | Hydroxylation, conjugation (glucuronidation, sulfation)[1] |
| Elimination half-life | IM: 40–50 days[1] |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C32H47F5O3S |
| Molar mass | 606.78 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fulvestrant, sold under the brand name Faslodex among others, is a medication used to treat breast cancer.[2] Specifically it is used for hormone receptor (HR) positive cases.[4][5] It is given by injection into a muscle.[2]
Common side effects include nausea, diarrhea, headache, pain, hot flushes, cough, swelling, rash, and trouble sleeping.[2] Other side effects may include low white blood cells, low red blood cells, low platelets, infection, and liver problems.[5] It should not be used in pregnancy or breastfeeding.[5] It is a selective estrogen receptor degrader (SERD) which binds to estrogen receptor resulting in their destruction.[6]
Fulvestrant was approved for medical use in the United States in 2002 and Europe in 2004.[2][5] It is available as a generic medication.[3] In the United Kingdom it costs the NHS about £520 per dose of 2021.[3] This amount in the United States costs about 100 USD.[7]
References[edit]
- ^ a b c d Dörwald FZ (4 February 2013). Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds. John Wiley & Sons. pp. 486–. ISBN 978-3-527-64565-7. Archived from the original on 10 October 2021. Retrieved 19 September 2021.
- ^ a b c d e f "Fulvestrant". The American Society of Health-System Pharmacists. Archived from the original on 2 February 2017. Retrieved 8 January 2021.
- ^ a b c BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 996. ISBN 978-0857114105.
- ^ "DailyMed - FULVESTRANT injection, solution". dailymed.nlm.nih.gov. Archived from the original on 12 December 2021. Retrieved 12 December 2021.
- ^ a b c d "Faslodex". Archived from the original on 19 October 2021. Retrieved 12 December 2021.
- ^ Lai AC, Crews CM (February 2017). "Induced protein degradation: an emerging drug discovery paradigm". Nature Reviews. Drug Discovery. 16 (2): 101–114. doi:10.1038/nrd.2016.211. PMC 5684876. PMID 27885283.
- ^ "Faslodex Prices and Faslodex Coupons - GoodRx". GoodRx. Retrieved 12 December 2021.
