User:Mr. Ibrahem/Finerenone
 | |
| Clinical data | |
|---|---|
| Trade names | Kerendia |
| Other names | BAY 94-8862 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Potassium-sparing diuretic |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
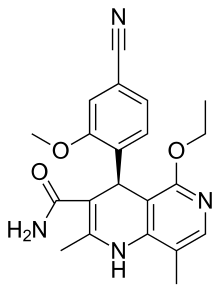
| Formula | C21H22N4O3 |
| Molar mass | 378.432 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Finerenone, sold under the brand name Kerendia, is a medication used to treat chronic kidney disease in people with type 2 diabetes.[6] It is used in those with moderate to severe disease with protein in the urine.[6] It is taken by mouth.[6]
Common side effects include high potassium, low blood pressure, and low sodium.[4] It should not be used in people with adrenal insufficiency.[4] It may interact with grapefruit.[4] There are concerns that use in pregnancy may harm the baby.[4] It is a non-steroidal mineralocorticoid receptor antagonist (MRA).[4]
Finerenone was approved for medical use in the United States in 2021 and Europe in 2022.[4][6] In the United Kingdom 4 weeks of medication costs the NHS about £52 as of 2022.[7] This amount in the United States is about 560 USD.[8]
References[edit]
- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Archived from the original on 3 April 2022. Retrieved 13 May 2022.
- ^ a b "Kerendia APMDS". Therapeutic Goods Administration (TGA). 9 December 2021. Archived from the original on 13 June 2022. Retrieved 12 June 2022.
- ^ "AusPAR: Finerenone". Therapeutic Goods Administration (TGA). 31 May 2022. Retrieved 12 June 2022.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c d e f g h i j "Kerendia- finerenone tablet, film coated". DailyMed. Archived from the original on 21 August 2021. Retrieved 20 August 2021.
- ^ "FDA Approves Drug for Chronic Kidney Disease". U.S. Food and Drug Administration (FDA). 9 July 2021. Archived from the original on 9 July 2021. Retrieved 9 July 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f "Kerendia EPAR". European Medicines Agency (EMA). 14 December 2021. Archived from the original on 16 March 2022. Retrieved 11 March 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Finerenone". SPS - Specialist Pharmacy Service. 7 January 2016. Archived from the original on 3 March 2022. Retrieved 29 October 2022.
- ^ "Kerendia". Archived from the original on 29 October 2022. Retrieved 29 October 2022.
