User:Mr. Ibrahem/Ezetimibe
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛˈzɛtɪmɪb, -maɪb/ |
| Trade names | Zetia, Ezetrol, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| Drug class | Cholesterol absorption inhibitor |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 35% to 65% |
| Protein binding | >90% |
| Metabolism | Intestinal wall, liver |
| Elimination half-life | 19 h to 30 h |
| Excretion | Renal 11%, faecal 78% |
| Identifiers | |
| |
| Chemical and physical data | |
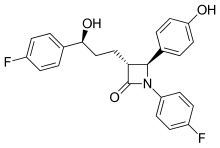
| Formula | C24H21F2NO3 |
| Molar mass | 409.433 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 164 to 166 °C (327 to 331 °F) |
| |
| |
| (verify) | |
Ezetimibe is a medication used to treat high blood cholesterol and certain other lipid abnormalities.[2] Generally it is used together with dietary changes and a statin.[3] Alone, it is less preferred than a statin.[2] It is taken by mouth.[2] It is also available in the fixed combinations ezetimibe/simvastatin, ezetimibe/atorvastatin, and ezetimibe/rosuvastatin.[2]
Common side effects include upper respiratory tract infections, joint pain, diarrhea, and tiredness.[2] Serious side effects may include anaphylaxis, liver problems, depression, and muscle breakdown.[2][3] Use in pregnancy and breastfeeding is of unclear safety.[4] Ezetimibe works by decreasing cholesterol absorption in the intestines.[3]
Ezetimibe was approved for medical use in the United States in 2002.[2] It is available as a generic medication.[3] A month supply in the United Kingdom costs the NHS about £26.21 as of 2019[update].[3] In the United States the wholesale cost of this amount is about US$5.85.[5] In 2017, it was the 151st most commonly prescribed medication in the United States, with more than four million prescriptions.[6][7]
References[edit]
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 9 September 2020.
- ^ a b c d e f g "Ezetimibe Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ a b c d e British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 196. ISBN 9780857113382.
- ^ "Ezetimibe (Zetia) Use During Pregnancy". Drugs.com. Retrieved 13 April 2019.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- ^ "Ezetimibe - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
