User:Mr. Ibrahem/Cevimeline
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | se vim' e leen[1] |
| Trade names | Evoxac |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608025 |
| Pregnancy category |
|
| Routes of administration | By mouth (capsules) |
| Drug class | Muscarinic agonist[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | <20% |
| Identifiers | |
| |
| Chemical and physical data | |
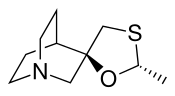
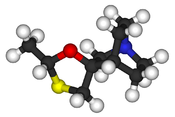
| Formula | C10H17NOS |
| Molar mass | 199.31 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cevimeline, sold under the brand name Evoxac, is a medication used to treat dry mouth due to Sjögren's syndrome or radiation therapy.[1] It is similar to pilocarpine.[2] It is taken by mouth.[1]
Side effects are usually mild and may include increased sweating, runny nose, nausea, diarrhea, headaches, dizziness, visual disturbances, and tiredness.[1] Safety in pregnancy is unclear.[2] It is a muscarinic agonist, which results in increased saliva production.[1]
Cevimeline was approved for medical use in the United States in 2000.[2] It is available as a generic medication.[3] In the United States a month of medication costs about 52 USD as of 2021.[3]
References[edit]
- ^ a b c d e f g h "Cevimeline". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 3 January 2022.
- ^ a b c "Cevimeline Monograph for Professionals". Drugs.com. Retrieved 3 January 2022. Cite error: The named reference "AHFS2022" was defined multiple times with different content (see the help page).
- ^ a b "Cevimeline Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 26 September 2016. Retrieved 3 January 2022.
