User:Mr. Ibrahem/Butorphanol
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | bue tor’ fa nol[1] |
| Trade names | Stadol, others |
| Other names | BC 2627 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682667 |
| Pregnancy category |
|
| Routes of administration | IV, intranasal |
| Drug class | Opioid agonist–antagonist[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Nasal: 60-70%; Sublingual: 25%-35%; PO 10% |
| Metabolism | Liver hydroxylated & glucuronidated |
| Onset of action | Within 15 min[2] |
| Elimination half-life | 4-7 hours |
| Duration of action | Up to 5 hrs[2] |
| Excretion | Kidney, 75% Biliary, 11-14% Fecal, 15% |
| Identifiers | |
| |
| Chemical and physical data | |
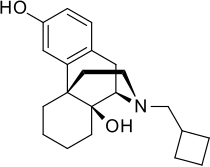
| Formula | C21H29NO2 |
| Molar mass | 327.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Butorphanol, sold under the brand name Stadol among others, is an opioid used to treat pain.[1] As a nasal spray, it may be used to treat migraines.[1] By injection into a vein or muscle, it may be used to treat moderate to severe pain.[1][2] Onset of effects is within 15 minutes and may last up to 5 hours.[2]
Side effects may include sleepiness, respiratory depression, confusion, feeling high, agitation, itchiness, sweating, nausea, and constipation.[1] While there is the potential for misuse, this is less than morphine.[1] Long term use in pregnancy can result in neonatal abstinence syndrome in the baby.[3] It has agonist–antagonist effects at the µ and ĸ type opiate receptors.[1]
Butorphanol was patented in 1971 and approved for medical use in 1978.[4][1] It is available as a generic medication.[1] In the United States a 1 mg vial for injection costs about 6 USD. [5] In the United States it is a schedule IV controlled substance.[1]
References[edit]
- ^ a b c d e f g h i j k l m "Butorphanol". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 15 May 2021. Retrieved 12 January 2022.
- ^ a b c d e "Butorphanol Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 12 January 2022.
- ^ "Butorphanol (Stadol) Use During Pregnancy". Drugs.com. Archived from the original on 29 October 2020. Retrieved 12 January 2022.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 529. ISBN 978-3-527-60749-5. Archived from the original on 2021-06-14. Retrieved 2021-10-21.
- ^ "Butorphanol Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 12 January 2022.
