User:Mr. Ibrahem/Binimetinib
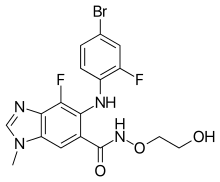 | |
| Clinical data | |
|---|---|
| Trade names | Mektovi |
| Other names | MEK162, ARRY-162, ARRY-438162 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618041 |
| License data |
|
| Drug class | Antineoplastic |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C17H15BrF2N4O3 |
| Molar mass | 441.233 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Binimetinib, sold under the brand name Mektovi, is as medication used to treat melanoma.[1] Specifically it is used with encorafenib for cases that are BRAF V600 positive and cannot be removed by surgery.[1] It is taken by mouth.[3]
Common side effects include tiredness, nausea, diarrhea, and abdominal pain.[2] Other side effects may include heart damage, blood clots, eye problems, interstitial lung disease, liver problems, muscle breakdown, and bleeding.[2] Use in pregnancy may harm the baby.[2] It works by blocking MEK, preventing its activation by BRAF, thereby slowing cancer growth.[1]
Binimetinib was approved for medication use in the United States and Europe in 2018.[2][1] In the United Kingdom 4 weeks of treatment costs the NHS about £4,500 as of 2021.[3] This amount in the United States costs about 12,800 USD.[4]
References[edit]
- ^ a b c d e "Mektovi". Archived from the original on 6 October 2021. Retrieved 10 January 2022.
- ^ a b c d e "DailyMed - MEKTOVI- binimetinib tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 6 April 2021. Retrieved 10 January 2022.
- ^ a b c BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1014. ISBN 978-0857114105.
- ^ "Mektovi Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 10 January 2022.
