User:Mr. Ibrahem/Aspirin
Template:Good article is only for Wikipedia:Good articles.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | acetylsalicylic acid /əˌsiːtəlˌsælɪˈsɪlɪk/ |
| Trade names | Bayer Aspirin, many others |
| Other names | 2-acetoxybenzoic acid acetylsalicylate acetylsalicylic acid O-acetylsalicylic acid, Aspirin (BAN UK), Aspirin (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682878 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, lysine acetylsalicylate may be given intravenously or intramuscularly |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80–100%[2] |
| Protein binding | 80–90%[3] |
| Metabolism | Liver, (CYP2C19 and possibly CYP3A), some is also hydrolysed to salicylate in the gut wall.[3] |
| Elimination half-life | Dose-dependent; 2 h to 3 h for low doses (100 mg or less), 15 h to 30 h for large doses.[3] |
| Excretion | Urine (80–100%), sweat, saliva, feces[2] |
| Identifiers | |
| |
| Chemical and physical data | |
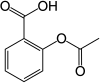
| Formula | C9H8O4 |
| Molar mass | 180.159 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.40 g/cm3 |
| Melting point | 136 °C (277 °F) [4] |
| Boiling point | 140 °C (284 °F) (decomposes) |
| Solubility in water | 3g/L |
| |
| |
| (verify) | |
Aspirin, also known as acetylsalicylic acid (ASA), is a medication used to reduce pain, fever, or inflammation.[5] Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever.[5] Aspirin given shortly after a heart attack decreases the risk of death.[5] Aspirin is also used long-term to help prevent further heart attacks, ischaemic strokes, and blood clots in people at high risk.[5] It may also decrease the risk of certain types of cancer, particularly colorectal cancer.[9] For pain or fever, effects typically begin within 30 minutes.[5] Aspirin is a nonsteroidal anti-inflammatory drug (NSAID) and works similarly to other NSAIDs but also suppresses the normal functioning of platelets.[5]
One common side effect is an upset stomach.[5] More significant side effects include stomach ulcers, stomach bleeding, and worsening asthma.[5] Bleeding risk is greater among those who are older, drink alcohol, take other NSAIDs, or are on other blood thinners.[5] Aspirin is not recommended in the last part of pregnancy.[5] It is not generally recommended in children with infections because of the risk of Reye syndrome.[5] High doses may result in ringing in the ears.[5]
A precursor to aspirin found in leaves from the willow tree has been used for its health effects for at least 2,400 years.[10][11] In 1853, chemist Charles Frédéric Gerhardt treated the medicine sodium salicylate with acetyl chloride to produce acetylsalicylic acid for the first time.[12] For the next fifty years, other chemists established the chemical structure and came up with more efficient production methods.[12]: 69–75 In 1897, scientists at the Bayer company began studying acetylsalicylic acid as a less-irritating replacement medication for common salicylate medicines.[12]: 69–75 [13] By 1899, Bayer had named it "Aspirin" and sold it around the world.[14] Aspirin's popularity grew over the first half of the twentieth century leading to competition between many brands and formulations.[15] The word Aspirin was Bayer's brand name; however, their rights to the trademark were lost or sold in many countries.[15]
Aspirin is one of the most widely used medications globally, with an estimated 40,000 tonnes (44,000 tons) (50 to 120 billion pills) consumed each year.[10][16] It is on the World Health Organization's List of Essential Medicines.[17] As of 2014[update], the wholesale cost in the developing world is US$0.002 to US$0.025 per dose.[18] As of 2015[update], the cost for a typical month of medication in the United States is less than US$25.00.[19] It is available as a generic medication.[5] In 2017, it was the 42nd most commonly prescribed medication in the United States, with more than 17 million prescriptions.[20][21]
References[edit]
- ^ a b "Aspirin Use During Pregnancy". Drugs.com. 2 April 2018. Archived from the original on 12 October 2019. Retrieved 29 December 2019.
- ^ a b "Zorprin, Bayer Buffered Aspirin (aspirin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 7 April 2014. Retrieved 3 April 2014.
- ^ a b c Brayfield, A, ed. (14 January 2014). "Aspirin". Martindale: The Complete Drug Reference. Pharmaceutical Press. Archived from the original on 13 July 2019. Retrieved 3 April 2014.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.8. ISBN 1-4398-5511-0.
- ^ a b c d e f g h i j k l m n o "Aspirin". Drugs.com. American Society of Health-System Pharmacists. 6 June 2016. Archived from the original on 25 April 2017. Retrieved 30 August 2016.
- ^ "ACETYLSALICYLIC acid = ASPIRIN = ASA oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 27 August 2021. Retrieved 25 August 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 17 September 2020. Retrieved 21 September 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 17 September 2020. Retrieved 21 September 2020.
- ^ Patrignani P, Patrono C (August 2016). "Aspirin and Cancer". Journal of the American College of Cardiology. 68 (9): 967–76. doi:10.1016/j.jacc.2016.05.083. PMID 27561771.
- ^ a b Jones, Alan (2015). Chemistry: An Introduction for Medical and Health Sciences. John Wiley & Sons. pp. 5–6. ISBN 978-0-470-09290-3.
- ^ Ravina, Enrique (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 24. ISBN 978-3-527-32669-3. Archived from the original on 21 February 2021. Retrieved 7 August 2020.
- ^ a b c Jeffreys, Diarmuid (2008). Aspirin the remarkable story of a wonder drug. Bloomsbury Publishing USA. ISBN 978-1-59691-816-0. Archived from the original on 8 September 2017.: 46–48
- ^ Dick, Brian (2018). "Hard Work and Happenstance". Distillations. Vol. 4, no. 1. Science History Institute. pp. 44–45. Archived from the original on 12 November 2020. Retrieved July 11, 2018.
- ^ Mann, Charles C.; Plummer, Mark L. (1991). The aspirin wars : money, medicine, and 100 years of rampant competition (1st ed.). New York: Knopf. p. 27. ISBN 978-0-394-57894-1.
- ^ a b "Aspirin". Chemical & Engineering News. Archived from the original on 11 October 2008. Retrieved 2007-08-13.
- ^ Warner TD, Mitchell JA (October 2002). "Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum?". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13371–3. Bibcode:2002PNAS...9913371W. doi:10.1073/pnas.222543099. PMC 129677. PMID 12374850.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Acetylsalicylic Acid". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 30 August 2016.
- ^ Hamilton, Richart (2015). Tarascon pocket pharmacopoeia (2015 deluxe lab-coat ed.). Jones & Bartlett Learning. p. 5. ISBN 978-1-284-05756-0.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ^ "Aspirin - Drug Usage Statistics". ClinCalc. Archived from the original on 20 October 2020. Retrieved 11 April 2020.
