User:Mr. Ibrahem/Altretamine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | al tret' a meen[1] |
| Trade names | Hexalen |
| Other names | Hexamethylmelamine, 2,4,6-Tris(dimethylamino)-1,3,5-triazine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601200 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (capsules) |
| Drug class | Alkylating agent[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 94% |
| Metabolism | Extensive liver |
| Metabolites | Pentamethylmelamine, tetramethylmelamine |
| Elimination half-life | 4.7–10.2 hours |
| Identifiers | |
| |
| Chemical and physical data | |
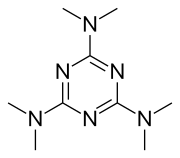
| Formula | C9H18N6 |
| Molar mass | 210.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Altretamine, sold under the brand name Hexalen, is a medication used to treat ovarian cancer.[1] Specifically it is used for advanced disease when other treatments are not effective.[1] It is taken by mouth.[1]
Common side effects include nausea, vomiting, diarrhea, hair loss, bone marrow suppression, peripheral nerve problems, and rash.[1] Other side effects may include mood disorders and further cancer.[2] Use in pregnancy may harm the baby.[2] It is an alkylating agent.[1]
Altretamine was approved for medical use in the United States in 1990.[1] As of 2022 it is not commercially available in the United States.[3]
References[edit]
- ^ a b c d e f g h i j "Altretamine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 14 January 2022.
- ^ a b "Altretamine Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 14 January 2022.
- ^ "Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. Archived from the original on 27 August 2021. Retrieved 14 January 2022.
