User:Lfarhat/sandbox
| RNA helicase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.6.4.13 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
A helicase in general is defined as “any of various enzymes that catalyze the unwinding and separation of double-stranded DNA or RNA during its replication”[1]. A helicase is more specifically considered a motor enzyme which receives its energy from NTP hydrolysis to unwind double stranded nucleic acids [2]. In our case we are considering specifically RNA Helicase which is involved holistically in the metabolism of RNA which is found in all facets of life on earth [3]. RNA helicase (EC 3.6.4.13, CSFV NS3 helicase, DBP2, DbpA, DDX17, DDX25, DDX3, DDX3X, DDX3Y, DDX4, DDX5, DEAD-box protein DED1, DEAD-box RNA helicase, DEAH-box protein 2, DEAH-box RNA helicase, DED1, Dex(H/D) RNA helicase, EhDEAD1, EhDEAD1 RNA helicase, eIF4A helicase, KOKV helicase, Mtr4p, nonstructural protein 3 helicase, NPH-II, RHA, RNA helicase A, RNA helicase DDX3, RNA helicase Hera, RNA-dependent ATPase, TGBp1 NTPase/helicase domain, VRH1, GRTH/DDX25) is an enzyme with system name ATP phosphohydrolase (RNA helix unwinding).[4][5][6][7][8][9][10][11] This enzyme catalyses the following chemical reaction
- ATP + H2O ADP + phosphate
RNA helicases utilize the energy from ATP hydrolysis to unwind RNA.
Structure and Superfamilies[edit]
RNA helicases are split into two main categories based on their ability to form oligomeric structures. Of the six superfamilies (SFs) that exist, SFs 1 and 2 do not form rings, whereas SFs 3, 4, 5, and 6 do.
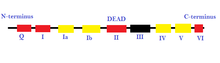
The first two superfamilies, which are usually found in eukaryotes, are comprised of a structurally conserved core that is usually surrounded by large N- and C- terminal domains that function as RNA and DNA binding domains, protein binding domains, and other molecular specific functions. The function of these domains is extremely significant in cellular interaction by increasing specificity of recruitment of proteins by using structurally specific complexes and sequences within families. These two helicases are also made up of at least 12 structural motifs are positioned in specific sequences that vary between families but are usually highly conserved within the same family [12].
SFs 3 through 6 form hexameric rings, and are usually found in bacteria and viruses [12]. Proteins of the superfamilies 3 and 4 are most similar to each other. However, SF4 contains a packaging motor named P4 that plays a role of packaging the RNA into a phage by first unwinding the structure and translocating the information into capsids. SF5 contains a Bacterial Rho factor that works to regulate transcription termination as well as removing RNA polymerase. SF6 contains a structure that works similarly to the Bacterial Rho factor but is different structurally [3].
Mechanisms[edit]
There are two known mechanisms for RNA helicase unwinding: canonical duplex unwinding and unwinding by local strand separation.
During canonical duplex unwinding, the helicase first binds to the single stranded region, then uses ATP hydrolysis as a power stroke in order to translocate the helicase across the strand. As the helicase slides down one strand, it is dissociating the two strands and removing the complementary. Finally, the helicase is removed.

The second unwinding mechanism is dependent on specific sequences called DEAD box helicases. As opposed to the canonical unwinding, local strand separation loads the helicase directly on the duplex region. DEAD box proteins, which are part of SF2, catalyze ATP-driven structural changes in RNA by unwinding substrates with the help of promoter sequences and accessory domains [13]. Although both mechanisms require ATP, local strand separation does not need to hydrolyze ATP as long as the ATP binds to the duplex strands [14].
Diseases Related to RNA Helicase[edit]
Most viruses are RNA viruses, usually containing an RNA helicase of their own (except in the case of retroviruses). These viruses depend on RNA helicase in order to replicate in the host, and helicase aids in transcription, translation, splicing, assembly, etc. in infectious diseases such as Hepatitis C. RNA processing has also played in a role in neurological disorders such as Amyotrophic lateral sclerosis (ALS, Lou Gehrig’s disease) and Alzheimer disease (AD). Alternative splicing that is carried out by the RNA helicase plays a central role in the nervous system, so defects in spliced mRNA may lead to serious degenerative effects in the spinal cord and brain. Especially at a young age, it is crucial for the transcription of mRNA to be responsive to binding factors for RNA helicase because these signals are essential for proper growth. It has also been shown that there are several RNA helicases that have altered gene expression in cancer cells. A specific helicase DDX1 is involved in mRNA processing and translation that affects cellular proliferation and tumor development. During the progression of cancer, tumor cells are growing at an increased rate by maintaining higher mRNA processing functions because of defects in DDX1. It is known that RNA helicases are extremely important for RNA metabolism, so problems in their function can be detrimental to human life. Antiviral and anti-cancer therapies have been targeting defects in RNA helicase and the results have been promising thus far [15].
See also[edit]
References[edit]
- ^ Merriam-Webster. Merriam-Webster, n.d. Web. 15 Oct. 2014.
- ^ Kwong, Ann D., Govinda B. Rao, and Kuan-Teh Jeang. "Viral and Cellular RNA Helicases as Antiviral Targets." Nature.com. Nature Publishing Group, Oct. 2005. Web. 15 Oct. 2014.
- ^ a b Jankowsky, Eckhard. RNA Helicases. Cambridge: Royal Society of Chemistry, 2010. Print.
- ^ Cordin, O., Tanner, N.K., Doere, M., Linder, P. and Banroques, J. (2004). "The newly discovered Q motif of DEAD-box RNA helicases regulates RNA-binding and helicase activity". EMBO J. 23: 2478–2487. doi:10.1038/sj.emboj.7600272. PMID 15201868.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rodamilans, B. and Montoya, G. (2007). "Expression, purification, crystallization and preliminary X-ray diffraction analysis of the DDX3 RNA helicase domain". Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63: 283–286. doi:10.1107/s1744309107006434. PMID 17401195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lee, C.G. and Hurwitz, J. (1992). "A new RNA helicase isolated from HeLa cells that catalytically translocates in the 3′ to 5′ direction". J. Biol. Chem. 267: 4398–4407. PMID 1537828.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li, S.C., Chung, M.C. and Chen, C.S. (2001). "Cloning and characterization of a DEAD box RNA helicase from the viable seedlings of aged mung bean". Plant Mol. Biol. 47: 761–770. PMID 11785937.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wu, J., Bera, A.K., Kuhn, R.J. and Smith, J.L. (2005). "Structure of the Flavivirus helicase: implications for catalytic activity, protein interactions, and proteolytic processing". J. Virol. 79: 10268–10277. doi:10.1128/jvi.79.16.10268-10277.2005. PMID 16051820.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gross, C.H. and Shuman, S. (1998). "The nucleoside triphosphatase and helicase activities of vaccinia virus NPH-II are essential for virus replication". J. Virol. 72: 4729–4736. PMID 9573237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Frick, D.N. (2007). "The hepatitis C virus NS3 protein: a model RNA helicase and potential drug target". Curr. Issues Mol. Biol. 9: 1–20. PMID 17263143.
- ^ Ivanov, K.A. and Ziebuhr, J. (2004). "Human coronavirus 229E nonstructural protein 13: characterization of duplex-unwinding, nucleoside triphosphatase, and RNA 5′-triphosphatase activities". J. Virol. 78: 7833–7838. doi:10.1128/jvi.78.14.7833-7838.2004. PMID 15220459.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jankowsky, Eckhard. "RNA Helicases at Work: Binding and Rearranging." Trends in Biochemical Sciences 36.1 (2011): 19-29. Web.
- ^ Yang, Quansheng, Mark Del Campo, Alan M. Lambowitz, and Eckhard Jankowsky. "DEAD-Box Proteins Unwind Duplexes by Local Strand Separation." Molecular Cell 28.2 (2007): 253-63. Web.
- ^ Jankowsky, Eckhard. RNA Helicases. Cambridge: Royal Society of Chemistry, 2010. Print.
- ^ Steimer, Lenz, and Dagmar Klostermeier. "RNA Helicases in Infection and Disease."RNA Biology 9.6 (2012): 751-71. Web.
External links[edit]
- RNA+helicase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
Category:EC 3.6.4 Lfarhat (talk) 01:18, 16 October 2014 (UTC) Sandbox for RNA Helicase Lfarhat (talk) 19:06, 21 October 2014 (UTC) Lfarhat (talk) 03:59, 4 November 2014 (UTC) Zeze bazzi (talk) 04:00, 4 November 2014 (UTC)


