User:Kkyann27/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /traɪˈmɛθəprɪm/ |
| Trade names | Proloprim, Monotrim, Triprim, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684025 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–100% |
| Protein binding | 44% |
| Metabolism | hepatic |
| Elimination half-life | 8-12 hours |
| Excretion | Urine (50–60%), faeces (4%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
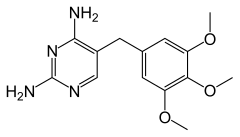
| Formula | C14H18N4O3 |
| Molar mass | 290.32 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
[1] Trimethoprim (TMP) is an antibiotic used [2]mainly in the treatment of urinary tract infections.[3] Other uses include for middle ear infections and travelers' diarrhea. With sulfamethoxazole or dapsone it may be used for Pneumocystis pneumonia in people with HIV/AIDS. It is taken by mouth.[3]
Common side effects include nausea, changes in taste, and rash. Rarely it may result in blood problems such as not enough platelets or white blood cells. May cause sun sensitivity.[3] There is evidence of potential harm during pregnancy in some animals but not humans.[4] It works by blocking folate metabolism in some bacteria which results in their death.[3]
Trimethoprim was first used in 1962.[5] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[6] It is available as a generic medication and is not very expensive.[7] In the United States 10 days of treatment is about 21 USD.[3]
Medical uses[edit]
It is primarily used in the treatment of urinary tract infections, although it may be used against any susceptible aerobic bacterial species.[8] It may also be used to treat and prevent Pneumocystis jiroveci pneumonia.[8] It is generally not recommended for the treatment of anaerobic infections such as Clostridium difficile colitis (the leading cause of antibiotic-induced diarrhoea).[8]
Co-trimoxazole[edit]
Trimethoprim was commonly (from 1969 to 1980 in the UK) used in a 1:5 combination with sulfamethoxazole, a sulfonamide antibiotic, which inhibits an earlier step in the folate synthesis pathway. This combination, also known as co-trimoxazole, TMP-sulfa, or TMP-SMX, results in an in vitro synergistic antibacterial effect by inhibiting successive steps in folate synthesis. This claimed benefit was not seen in general clinical use.[9][10]
The combination's use has been declining due to reports of sulfamethoxazole having bone marrow toxicity, resistance and lack of greater efficacy in treating common urinary and chest infections,[11][12][13][14] and side effects of antibacterial sulfonamides. As a consequence, the use of co-trimoxazole was restricted in 1995 [15] following the availability of trimethoprim (not in combination) in 1980.
With its greater efficacy against a limited number of bacteria, co-trimoxazole remains indicated for some infections; for example, it is used as prophylaxis in patients at risk for Pneumocystis jirovecii pneumonia (e.g. AIDS patients and those with some hematological malignancies) and as therapy in Whipple's disease. Gram-positive bacteria are generally or moderately susceptible.
Susceptibility[edit]
| Micro-organism name | Susceptible to trimethoprim?[8] |
|---|---|
| Aerobic bacteria | |
| Acinetobacter sp. | No |
| Aeromonas sp. | Yes |
| Burkholderia cepacia | Yes |
| Burkholderia pseudomallei | No |
| Campylobacter coli | No |
| Campylobacter jejuni | No |
| Citrobacter freundii | Yes |
| Corynebacterium jeikeium | No |
| Enterobacter sp. | Yes |
| Enterococcus sp. | No |
| Escherichia coli | Yes |
| Haemophilus influenzae | Yes |
| Klebsiella sp. | Yes |
| Moraxella catarrhalis | No |
| Morganella sp. | Yes |
| Neisseria meningitidis | No |
| Proteus mirabilis | Yes |
| Proteus vulgaris | Yes |
| Providencia sp. | Yes |
| Pseudomonas aeruginosa | No |
| Salmonella sp. | Yes |
| Serratia sp. | Yes |
| Shigella sp. | No |
| Staphylococcus aureus | Yes |
| Staphylococcus saprophyticus | Yes |
| Stenotrophomonas maltophilia | No |
| Streptococcus - groups A, B, C, G | Yes |
| Streptococcus pneumoniae | No |
| Viridans streptococci | No |
| Yersinia sp. | Yes |
Side effects[edit]
Trimethoprim can cause thrombocytopenia (low levels of platelets) by lowering folic acid levels; this may also cause megaloblastic anemia. Trimethoprim antagonises the epithelial sodium channel in the distal tubule, thus acting like amiloride. This can cause hyperkalemia. Trimethoprim also competes with creatinine for secretion into the renal tubule. This can cause an artefactual rise in the serum creatinine. Use in EHEC infections may lead to an increase in expression of Shiga toxin.[16] Because it crosses the placenta and can affect folate metabolism, trimethoprim is relatively contraindicated during pregnancy, especially the first trimester.[17] It may be involved in a reaction similar to disulfiram when alcohol is consumed after it is used, in particular when used in combination with sulfamethoxazole.[18][19] The trophoblasts in the early fetus are sensitive to changes in the folate cycle. A recent study has found a doubling in the risk of miscarriage in women exposed to trimethoprim in the early pregnancy.[20]
Mechanism of action[edit]

Trimethoprim binds to dihydrofolate reductase and inhibits the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF).[21] THF is an essential precursor in the thymidine synthesis pathway and interference with this pathway inhibits bacterial DNA synthesis.[21] Trimethoprim's affinity for bacterial dihydrofolate reductase is several thousand times greater than its affinity for human dihydrofolate reductase.[21] Sulfamethoxazole inhibits dihydropteroate synthetase, an enzyme involved further upstream in the same pathway.[21] Trimethoprim and sulfamethoxazole are commonly used in combination due to claimed synergistic effects,[21] and reduced development of resistance.[21] This benefit has been questioned.[9]

See also[edit]
References[edit]
- ^ "Affordable Care Act". www.medicaid.gov. Retrieved 2015-11-23.
- ^ "Costs of Care - Long-Term Care Information". longtermcare.gov. Retrieved 2016-01-15.
- ^ a b c d e "Trimethoprim". The American Society of Health-System Pharmacists. Retrieved Aug 1, 2015.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Retrieved 22 April 2014.
- ^ Huovinen, P (1 June 2001). "Resistance to trimethoprim-sulfamethoxazole". Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America. 32 (11): 1608–14. doi:10.1086/320532. PMID 11340533.
- ^ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 113. ISBN 9781284057560.
- ^ a b c d Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ a b Brumfitt, W; Hamilton-Miller, JM (December 1993). "Reassessment of the rationale for the combinations of sulphonamides with diaminopyrimidines". Journal of Chemotherapy. 5 (6): 465–9. doi:10.1080/1120009X.1993.11741097. PMID 8195839.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brumfitt W, Hamilton-Miller JM (February 1993). "Limitations of and indications for the use of co-trimoxazole". J Chemother. 6 (1): 3–11. doi:10.1080/1120009x.1994.11741120. PMID 8071675.
- ^ Bean DC, Livermore DM, Papa I, Hall LM (November 2005). "Resistance among Escherichia coli to sulphonamides and other antimicrobials now little used in man". J Antimicrob Chemother. 56 (5): 962–4. doi:10.1093/jac/dki332. PMID 16150859.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Felmingham D, Reinert RR, Hirakata Y, Rodloff A (September 2002). "Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and compatative in vitro activity of the ketolide, telithromycin". J Antimicrob Chemother. 50 (Suppl S1): 25–37. doi:10.1093/jac/dkf808. PMID 12239226.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Johnson JR, Manges AR, O'Bryan TT, Riley LW (June 29, 2002). "A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis". Lancet. 359 (9325): 2249–51. doi:10.1016/S0140-6736(02)09264-4. PMID 12103291. S2CID 46759747.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lawrenson RA, Logie JW (December 2001). "Antibiotic failure in the treatment of urinary tract infections in young women". J Antimicrob Chemother. 48 (6): 895–901. doi:10.1093/jac/48.6.895. PMID 11733475. - suggest some small advantage in UTIs
- ^ "Co-trimoxazole use restricted". Drug Ther Bull. 33 (12): 92–3. December 1995. doi:10.1136/dtb.1995.331292. PMID 8777892. S2CID 37853468.
- ^ Kimmitt PT, Harwood CR, Barer MR (2000). "Toxin Gene Expression by Shiga Toxin-producing Escherichia coli: The Role of Antibiotics and the Bacterial SOS Response". Emerg Infect Dis. 6 (5): 458–465. doi:10.3201/eid0605.000503. PMC 2627954. PMID 10998375.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Use extra precautions when taking the contraceptive pill". netdoctor.co.uk.
- ^ Edwards DL, Fink PC, van Dyke PO (1986). "Disulfiram-like reaction associated with intravenous trimethoprim-sulfamethoxazole and metronidazole". J Clinical Pharmacy. 5 (12): 999–1000. PMID 3492326.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Heelon MW; White M (1998). "Disulfiram cotrimoxazole reaction". J Pharmacotherapy. 18 (4): 869–870. doi:10.1002/j.1875-9114.1998.tb03913.x. PMID 9692665. S2CID 23968977.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Andersen JT, Petersen M, Jimenez-Solem E, Broedbaek K, Andersen EW, Andersen NL, Afzal S, Torp-Pedersen C, Keiding N, Poulsen HE (2013). "Trimethoprim use in early pregnancy and the risk of miscarriage: a register-based nationwide cohort study". Epidemiology and Infection. 141 (8): 1749–1755. doi:10.1017/S0950268812002178. PMID 23010291.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Brogden, RN; Carmine, AA; Heel, RC; Speight, TM; Avery, GS (June 1982). "Trimethoprim: a review of its antibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections". Drugs. 23 (6): 405–30. doi:10.2165/00003495-198223060-00001. PMID 7049657. S2CID 21806926.
- ^ Heaslet, H.; Harris, M.; Fahnoe, K.; Sarver, R.; Putz, H.; Chang, J.; Subramanyam, C.; Barreiro, G.; Miller, J. R. (2009). "Structural comparison of chromosomal and exogenous dihydrofolate reductase fromStaphylococcus aureusin complex with the potent inhibitor trimethoprim". Proteins: Structure, Function, and Bioinformatics. 76 (3): 706–717. doi:10.1002/prot.22383. PMID 19280600. S2CID 1373618.
External links[edit]
- Nucleic acid inhibitors (PDF file).
Category:Antibiotics Category:Bacterial dihydrofolate reductase inhibitors Category:Protozoal dihydrofolate reductase inhibitors Category:Pyrimidines Category:World Health Organization essential medicines Category:Phenol ethers Category:Aromatic amines
