User:Isabel.guillen.5/sandbox
| |
| Names | |
|---|---|
| IUPAC name
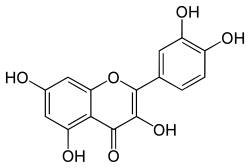
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one
| |
| Other names
Sophoretin
Meletin Quercetine Xanthaurine Quercetol Quercitin Quertine Flavin meletin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H10O7 | |
| Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder[1] |
| Density | 1.799 g/cm3 |
| Melting point | 316 °C |
| Practically insoluble in water; soluble in aqueous alkaline solutions[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Quercetin is the most common flavonoid in nature. It is found as its glycosylated forms such as quercitrin (3-rhamnosylquercetin) or rutoside (3-rhamnosy-glucosyl quercetin),[2] specifically as a flavonol, which is a polyphenolic compound synthesized in plant cells.[3] It exhibits antioxidant properties by reducing oxidative stress in cells via two distinct pathways: (1) radical scavenging activities and binding of transition metal ions and (2) competitively inhibiting xanthine oxidase (XOD).[4] Quercetin is familiarly used in traditional medicine to prevent or treat diseases such as cancer, cardiovascular and nervous diseases, obesity, and chronic inflammation. Quercetin is naturally found in fruits and vegetables, especially onions, citrus and apples. Other sources include dark berries, grapes and olive oil. Green tea and red wine because of their high percentage of antioxidants and flavonoids have been identified as having prominent amounts of quercetin. [5]
Properties[edit]
Antioxidant[edit]
Antioxidants are molecules that protect against the chain reaction known as oxidative stress caused by free radicals and reactive oxygen species (ROS). They give free radicals the electron they are searching for, stabilizing them before they damage essential macromolecules, such as DNA, proteins, lipids and carbohydrates. Quercetin has antioxidant properties exhibiting radical-scavenging activities that aid in the process of removing free radicals in biological organisms. The reductive properties of quercetin are attributed to the two phenolic hydroxyl groups, known as catechol group, which function as electron donating groups that are capable of being oxidized by free radicals. In addition, quercetin is classified as an antioxidant because it is capable of competitively inhibiting oxidative enzymes, such as xanthine oxidase, which generates superoxide molecules as a byproduct of converting xanthine to uric acid in the purine catabolic pathway, playing a key role in initiating radical-induced cellular damage in biological organisms. [6] Quercetin's antioxidant properties allow it to specifically inhibit lipid peroxidation. [7] As more in vitro studies are performed, there is increasing evidence that quercetin, as an antioxidant, protects cellular structures against oxidative damage and therefore, it can limit the risk of various degenerative diseases, such as, Alzheimer's, Parkinson's, Huntington's, and Amyotrophic lateral sclerosis, correlated to oxidative stress. [8]
Pro-oxidant[edit]
Pro-oxidants are molecules that promote oxidative stress by producing free radicals in a system and/or inhibiting antioxidant mechanisms. Studies have shown that quercetin has the potential to exhibit pro-oxidant properties and therefore cytotoxic effects on cells, depending on concentration and free radical source. [9] The enzymatically catalyzed oxidative degradation of quercetin produces o-semiquinone and o-quinone. o-semiquinone expedites the formation of superoxide while simultaneously decreasing glutathione (GSH). o-quinone's function is to increase the semiquinone supply in the reaction, therefore magnifying the pro-oxidative effect of quercetin. [10]
Biosynthesis[edit]
Quercetin is an organic compound chemically classified as a hydrocarbon, specifically as a flavonol. Quercetin is biosynthesized from dihydroquercetin, a type of flavonol that participates in a reaction catalyzed by flavonol synthase. Flavanols have a 2-hydroxyflavone (IUPAC name: 3-hydroxy-2-phenylchromen-4-one) general backbone structure and quercetin is distinguished from other flavonols by the distinct positioning of the two phenolic -OH groups giving it the structure 2-(3,4-dihydroxyphenyl)- 3,5,7-trihydroxy-4H-chromen-4-one. The nonpolar properties of quercetin are attributed to the hydrophobic, co-coplanar structure of the molecule, which becomes more soluble in an aqueous solution when the hydroxyl groups are substituted with sugars.
Pathway[edit]
The following steps details the biosynthesis pathway for quercetin in model plants, specifically, snapdragon, Arabidopsis, maize, and petunia. [11]
Step 1: Phenylalanine is converted to para-coumaryl CoA in a series of steps known as the general phenylpropanoid pathway using phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA-ligase.
Step 2: Chalcone synthase, a type III polyketide synthase, catalyzes the condensation of para-coumaryl CoA and three malonyl-CoA thioesters to give chalcone.
Step 3: Chalcone isomerase catalyzes the intramolecular cyclization of chalcone to give (2S)-naringenin, a flavanone derivative of many flavonoids.
Step 4: Flavanone 3β-hydroxylase (FHT) catalyzes the B-ring hydroxylation of naringenin to produce dihydrokaempferol, a type of dihydroflavanol.
Step 5: Dihydrokaempferol is converted to dihydroquercetin by the actions of Flavonoid 3′-hydroxylase.
Step 6: Flavonol synthase catalyzes the conversion of dihydroquercetin into quercetin.
Clinical Relevance[edit]
Anti-Cancer activity[edit]
Due to its antioxidant, anti-tumor and anti-inflammatory activity, quercetin has been studied extensively as a chemoprevention agent in several cancer models, since it is thought to prevent tumor angiogenesis. [12] Studies suggest that quercetin slow down the growth of cancer cells and help promote apoptosis.[13]In breast cancer cell lines, quercetin was able to inhibit the expression of mutant p53 protein to levels that were almost undetectable. By inhibiting the expression of p53 the cells arrest the cell cycle at the G2-M cell cycle check point and stop dividing.[14] Quercetin is able to inhibit production of heat shock proteins in many malignant cell lines such as breast cancer, leukemia, and colon cancer. Heat shock proteins allow tumor cells to bypass normal mechanisms of cell cycle arrest by forming a complex with mutant p53 and allows for an increased survival rate of cancer cells under different bodily stresses such as low circulation and high fever.[14]
Anti-Inflammatory activity[edit]
By inhibiting the cofactor recruitment at the chromatin of pro-inflammatory genes, quercetin exerts its anti-inflammatory effects in epithelial cells. [15] Studies suggest that quercetin can help stabilize the cells that release histamine in the body and therefore have an anti-inflammatory effect. [16]At high concentrations, quercetin, mainly because of its catechol group, was shown to block both the cyclooxygenase and lipooxygenase activity, both which are responsible for the inflammatory effect in cells. [17]
Fate in vivo[edit]
Following dietary ingestion, quercetin undergoes rapid and extensive metabolism that makes the biological effects presumed from in vitro studies unlikely to apply in vivo.[18][19][20] Rodents that were intravenously administered with the Quercetin Aglycone showed a rapid decrease in plasma quercetin concentrations and a lack of accumulation of quercetin in the tissues. [21] These results suggest that quercetin is quickly metabolized and excreted into urine following an intravenous injection of the compound in rodents.
Side Notes[edit]
Since quercetin is still being studied extensively in vitro, it has not been confirmed scientifically as a specific therapeutic for any condition nor approved by any regulatory agency. The European Food Safety Authority evaluated possible health claims associated with consumption of quercetin, finding that no cause-and-effect relationship has been established for any physiological effect.[22] Quercetin dietary supplements have been promoted for a claimed ability to prevent and treat cancer; however, according to the American Cancer society, "there is no reliable clinical evidence that quercetin can prevent or treat cancer in humans".[23] Only when quercetin is studied extensively in vivo is that data gathered will lead to direct predictions on its role in the human body.
References[edit]
- ^ a b c Quercetin dihydrate safety sheet on http://www.pvp.com.br (English)
- ^ Monica Comalada, Desire Camuesco, Saleta Sierra, Isabel Ballester, Jordi Xaus, Julio Galvez and Antonio Zarzuelo. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-jB pathway. European Journal of Immunology. <http://hera.ugr.es/doi/15772342.pdf>
- ^ Seong-Min Choi, Byeong C. Kim,corresponding author Yeun-Hee Cho, Kang-Ho Choi, Jane Chang, Man-Seok Park, Myeong-Kyu Kim, Ki-Hyun Cho, and Jong-Keun Kim. Effects of Flavonoid Compounds on β-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons. August 20, 2014. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4161760/>
- ^ Murakami, Akira (8 October 2008). [(http://www.sciencedirect.com/science/article/pii/S0304383508002607) "Multitargeted cancer prevention by quercetin"]. Cancer Letters. Volume 269 (Issue 2): Pages 315–325.<http://www.sciencedirect.com/science/article/pii/S0304383508002607>
- ^ <University of Maryland Medical Center. May 07, 2013 <http://umm.edu/health/medical/altmed/supplement/quercetin>
- ^ Murakami, Akira (8 October 2008 "Multitargeted cancer prevention by quercetin". Cancer Letters. Volume 269 (Issue 2): Pages 315–325. <http://www.sciencedirect.com/science/article/pii/S0304383508002607>
- ^ Bentz, Alexandra. "A Review of Quercetin: Chemistry, Antioxidant Properties, and Bioavailability" Journal of Young Investigators. April 2009. <http://www.jyi.org/issue/a-review-of-quercetin-chemistry-antioxidant-properties-and-bioavailability/>
- ^ Kanti Bhooshan Pandey and Syed Ibrahim Rizvi. "Plant polyphenols as dietary antioxidants in human health and disease". <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2835915/>
- ^ Guohua Cao, Emin Sofic, Ronald L. Prior. "Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships". Free Radical Biology and Medicine. Volume 22, Issue 5, 1997, Pages 749–760.<http://www.sciencedirect.com/science/article/pii/S0891584996003516>
- ^ Metodiewa D, Jaiswal AK, Cenas N, Dickancaité E, Segura-Aguilar J."Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product". <http://www.ncbi.nlm.nih.gov/pubmed/9890646>
- ^ B. Winkel-Shirley. "Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology". Plant Physiol. Jun 2001; 126(2): 485–493.<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1540115/>
- ^ University of Maryland Medical Center. May 07, 2013 <http://umm.edu/health/medical/altmed/supplement/quercetin
- ^ American Cancer Society. Quercetin.<http://www.cancer.org/treatment/treatmentsandsideeffects/complementaryandalternativemedicine/dietandnutrition/quercetin>
- ^ a b Lamson DW, Brignall MS (June 2000). "Antioxidants and cancer, part 3: quercetin". Altern Med Rev. 5 (3): 196–208. PMID 10869101.
{{cite journal}}: CS1 maint: date and year (link) - ^ Anti-inflammatory action of quercetin. Phytochemicals. <http://www.phytochemicals.info/phytochemicals/quercetin/anti-inflammatory.php>
- ^ Mona S. Mohammed, Wadah J.A. Osman, Elrashied A.E. Garelnabi, Zuheir Osman, Bashier Osman, Hassan S. Khalid, Magdi A. Mohamed. "Secondary metabolites as anti-inflammatory agents". The Journal of Phytopharmacology 2014; 3(4): 275-285. <http://www.phytopharmajournal.com/Vol3_Issue4_09.pdf>
- ^ Mona S. Mohammed, Wadah J.A. Osman, Elrashied A.E. Garelnabi, Zuheir Osman, Bashier Osman, Hassan S. Khalid, Magdi A. Mohamed. "Secondary metabolites as anti-inflammatory agents". The Journal of Phytopharmacology 2014; 3(4): 275-285. <http://www.phytopharmajournal.com/Vol3_Issue4_09.pdf>
- ^ Williams RJ, Spencer JP, Rice-Evans C (April 2004). "Flavonoids: antioxidants or signalling molecules?". Free Radical Biology & Medicine. 36 (7): 838–49. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gross P (March 1, 2009), New Roles for Polyphenols. A 3-Part report on Current Regulations & the State of Science, Nutraceuticals World
- ^ Barnes S, Prasain J, D'Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver CM (2011). "The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems". Food & Function. 2 (5): 235–244. doi:10.1039/c1fo10025d. PMC 4122511. PMID 21779561.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Murakami, Akira (8 October 2008). [(http://www.sciencedirect.com/science/article/pii/S0304383508002607) "Multitargeted cancer prevention by quercetin"]. Cancer Letters. 269 (2): 315–325. doi:10.1016/j.canlet.2008.03.046. PMID 18467024. Retrieved 5 December 2014.
{{cite journal}}: Check|url=value (help) - ^ "Scientific Opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), "cardiovascular system" (ID 1844), "mental state and performance" (ID 1845), and "liver, kidneys" (ID 1846) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 8 April 2011. Retrieved 24 September 2014.
- ^ "Quercetin". American Cancer Society. November 2008. Retrieved September 2013.
{{cite web}}: Check date values in:|accessdate=(help)
Category:Quercetin Category:Flavonoid antioxidants Category:Xanthine oxidase inhibitors Category:Experimental medical treatments

