User:Gpa4.30/sandbox
Opioid analgesics refer to opioid receptor agonists which produce a pain-killing effect. The earliest record of it could be found in 3500 B.C. when Sumerians used opium to relieve pain. Opioids can be classified to different classes by their chemical structures or agonistic effect. They produce the analgesic effect by activating various types of opioid receptors in both presynaptic and postsynaptic neurons to inhibit the transmission of pain signals.
In terms of pharmacokinetics, opioids could have significant drug-drug interactions due to their CYP3A and CYP2D6 metabolism. Some drugs could bind to the CYP enzyme and alter the metabolism of opioids. In terms of pharmacodynamics, opioids could interact with CNS depressants to provide a synergetic effect, which induces excessive depression and may lead to death.
Opioids are commonly used for moderate to severe pain relief in post-surgery patients or cancer patients. It could induce side effects in the central nervous system such as sedation, drowsiness and depression. Opioids could also lead to addiction, which makes opioid abuse become a significant health problem in society. To prevent opioid overdose, it is suggested to reduce the dose and duration prescribed to minimise its effect on the body.
History[edit]
Ancient time
The earliest recorded use of opioids to relieve pain can be traced back to 3400 to 3500 B.C. – the Sumerians in lower Mesopotamia. The Sumerians used opium, an intensely addictive compound derived from the opium poppy, to treat pain, induce sleep and produce euphoria[1] . Owing to its recreational and medical use, opium was linked to the God of sleep and death and soon spread to various ancient cultures along the Silk Road, such as Assyrians, Greeks and China, where opium was the underlying reason for the Opium Wars during the Qing Dynasty.
Industrial Revolution

The medical use of opioids in relieving pain was a breakthrough in the 19th century. In 1803, German pharmacist Friedrich Wilhelm Adam Serturner extracted morphine, the primary active ingredient in opium, and named it after Morpheus, the Greek god of dreams[1]. The extraction of opium had not come to an end; soon, more than 20 pharmacologically active alkaloids were found and isolated from opium. In 1853, a French physician, Charles-Gabriel Pravaz, introduced and modified the hypodermic syringe to inject drugs into the bloodstream directly[2]. By bypassing the digestive system, the injected morphine achieved maximum bioavailability which referred to the amount of drug that can reach the systemic circulation, the shortest onset time and a controlled dosage regimen. Later, in 1894, the Bayer pharmaceutical company modified morphine and its natural derivatives to synthesise diacetylmorphine and named it Heroin[1] . Initially, Bayers claimed that Heroin is a non-addictive analogue of morphine and can be used in clinical medicine to treat opioid addiction, which was found more addictive than morphine afterwards.
Opium War
Meanwhile. the UK government initiated a trilateral trade with the British Raj and the Qing Dynasty. The Qing goverment provided tea herbs to the Britans while the British Raj provided opium as medical and recreational goods to the Qing. Owing to the addictive feaures, opium soon created a massive hit within the Qing Dynasty, leading to a deteriorated army force and a vast outflow of wealth [3]. The governors of Qing Dynasty intended to cease the import of opium which may downregulate the trade benefit of the Britains. A politan named Lin ZeXu was sent to discard the imported opium in 1839 at Humen, this event - Destruction of Opium at Humen became the trigger of the Britans. As a result, the opium war started.
World Wars
Opioid analgesics use and demand continued to grow in the warfares. Morphine and cocaine were deployed in medical units during World Wars due to their analgesic and stimulant properties. They are commonly use in countries like France, German and Britain as they could be self-administered primarily before the field medic arrived to minimize the pain suffer by the soilders. In addition to morphine and cocaine, other opioid analgesics like methadone were also deployed in specific situations during WWII[4]; however, It is noteworthy that the use of opioid analgesics was closely monitored and regulated by medical professionals even in wartime to lower the risk of addiction and abuse.
Post Industrial-periods
People started to become aware of the problem brought on by opioid addiction. Opioid abuse did not cease after the outbreak of war; fentanyl and other derivatives were still undergoing development and released to the public. Later, the World Health Organization announced a new regulation guildine to classify and separate over-the-counter drugs and prescription drugs druing the 1950s[5]. To stop people from owning and selling opioids without instruction from healthcare professionals, criminal penaltis of minium sentences was setted up afterwards.

However, the limited access of opioid analgesics failed to suppress the huge demand and drawback of opioid analgesics. For instance, the fentanyl abuse has resulted in the most problematic opioid crisis in the United States. The number of fentanyl related emergency visit doubled over the 2010s and nearly 10k U.S. citizens died of the abuse of fentanyl only in 2015 which is nearly a double of the previous year[6]. This indicated that the opioid analgesics abuse kept worsening which is shown in the proximal image.
Classification[edit]
Opioid analgesics has two classification systems: structure and agonistic effects.
Structure[edit]
The structure of opioid analgesics falls into 4 main categories,[7]
- Phenanthrene derivatives
- Diphenylpropylamine derivatives
- Benzomorphan derivatives
- Phenylpiperidine derivatives

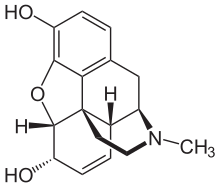
All phenanthrene-derived opioids have a rigid phenanthrene base and bind with both the Mu (µ) opioid receptor and the Kappa (κ) opioid receptor[7][8]. Phenenthrene-derived opioids can be naturally occurring or semi-synthetic. The naturally occurring opioids extracted from Papaveraceae plants all belong to this structure, including morphine, codeine and thebaine. Oxycodone and heroin are examples of semi-synthetic phenanthrene-derived opioids that are synthesised from morphine[9].
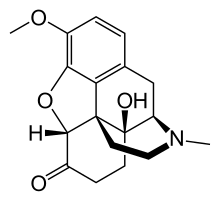
Diphenylpropylamine derivatives, Benzomorphan derivatives and Phenylpiperidine derivatives are all synthetic opioids. They are flexible opioids and they only bind with the Mu (µ) opioid receptor[8]. Diphenylpropylamine derivatives include methadone and propoxyphene. Benzomorphan derivatives include pentazocine and phenazocine. Phenylpiperidine derivatives can be further subdivided into two categories: meperidine(pethidine) and fentanyl derivatives. Fentanyl derivatives includes fentanyl, remifentanyl and alfentanil[7].
| Phenanthrene derivatives | Diphenylpropylamine derivatives | Benzomorphan derivatives | Phenylpiperidine derivatives |
|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Agonistic effect[edit]
Opioid analgesics can be classified into full agonists partial agonists, mixed agonists and antagonists.
Full Agonists[edit]
Full opioid agonists activate the opioid receptors and result in full opioid effects[19]. Examples of full opioid agonists include morphine, heroin and fentanyl. They have strong analgesic effect of even anaesthetic effect that can be used in severe pain management[20] and during surgery[21].
Partial Agonists[edit]

Partial opioid agonist activate the opioid receptor and result in much less opioid effects than full agonists[19]. One example of partial opioid agonists is Tramadol.
Mixed Agonists/ Antagonists[edit]
Mixed opioid agonist/antagonists activates certain opioid receptors and blocks the other opioid receptors. They generally have a lower risk of abuse and addiction compared to the full agonists[22]. Buprenorphine, Butorphanol, Nalbuphine and Pentazocine are some examples of mixed agonists/antagonists.
Antagonists[edit]
Opioid antagonists binds to the opioid receptors and produces zero effects. Naloxone and Naltrexone are the only two opioid anatagonist that is FDA approved to treat opioid overdose caused respiratory depression. It is recorded that over 26500 people were saved using naloxone to treat opioid overdose in the U.S[23].
Pharmacology[edit]
Opioid analgesics are opioid receptor agonists that bind to and activate opioid receptors in both presynaptic and postsynaptic neurons. In presynaptic neurons, the activation of opioid receptors triggers the release of G-beta-gamma complex, which then interact with the voltage-gated calcium channels.[24] This interaction inhibits the opening of the channels, preventing the influx of calcium ions into presynaptic neurons upon arrival of an action potential. As a result, the release of neurotransmitters, including glutamate, calcitonin, and substance P, is reduced, thus effectively blocking pain signalling transmission.[20]

In postsynaptic neurons, the activation of opioid receptors also leads to the release of G-beta-gamma subunits. The subunits would open G-protein-coupled potassium channels, causing the efflux of potassium ions. This depolarizes the neurons and counteract the hyperpolarisation induced by pain signals. Action potential becomes more difficult to generate within the neurons, thus inhibiting the transmission of pain signals.
While most opioid receptors can produce analgesic effects, their exact pharmacodynamic responses vary with the receptors they bind to. Mu receptors binding can produce various effects, including vomiting, sedation, and urinary retention, while Kappa receptors binding can contribute to respiratory depression, dysphoria, and dyspnea. On the other hand, Delta receptors binding mainly exert spinal analgesic effects. The affinity of drugs to these different receptors determines their specific side effects. For example, morphine, a strong agonist of Mu receptors, can result in sedation when patients take it.
Drug Interaction[edit]
Drug interactions can be further subdivided into two types of interaction: pharmacokinetic interaction and pharmacodynamic interaction
Pharmacokinetic Interaction[edit]
The main metabolism of opioid drugs is through the CYP450 enzyme[25]. The phase 1 metabolism of opioid drugs is mainly through CYP3A4 and CYP2D6 enzymes[26][27].
The majority of fentanyl, oxycodone, codeine and methadone undergoes phase 1 metabolism through CYP3A4 enzyme[26]. As for the CYP2D6 enzyme, it is responsible for the metabolism of codeine[26], with minor effects on oxycodone and methadone metabolism. Therefore, the CYP3A4 or CYP2D6 enzyme inhibitors will increase the serum concentration of certain opioid drugs which may lead to opioid overdose[27].
To prevent the overdose of opioids, naloxone can be co-prescribed with opioid analgesics[28]. Naloxone is an opioid receptor antagonist that can reverse the effect of opioids[29]. 0.04-2mg IV injection of naloxone can treat patients experiencing cardiac arrest or respiratory depression due to opioid toxicity[30].
Pharmacodynamic Interaction[edit]
CNS depressing effect is one of the main side effects of opioid analgesics, especially for the higher potency opioids like fentanyl and meperidine. It was reported that antihistamines were found in 14.7% of opioid overdose deaths in 2019 in the United States[31]. Moreover, diphenhydramine, a first-generation antihistamine which exerts a sedative effect, was related to more than 50% of antihistamine-related deaths. It was suggested that the CNS depressing effect of opioids synergised with the sedative effect of antihistamines, leading to respiratory depression and resulting in death[32].
CNS depressants like benzodiazepines and alcohol may also enhance the CNS depression effect of opioid analgesics. Therefore a synergetic effect will be created and the user may experience breath suppression which may result in death[33]. Moreover, a study in 2015 regarding the co-administration of benzodiazepines and opioid analgesics showed an increased use of emergency services by the patients[34]. Therefore generally opioid analgesics should not be co-prescribed with benzodiazepine anxiolytics like diazepam. Patients should also avoid consuming any alcohol when administering opioid analgesics as co-administration of opioids and alcohol leads to more than 60% of unintended overdoses and deaths[35].
The analgesic effects of opioids could also be synergised with NSAIDs (non-steroidal anti-inflammatory drugs) and paracetamol. Co-administration of NSAIDs and opioid analgesics may increase the analgesic effect and lead to a better pain-reliving effect[36]. The use of paracetamol could also decrease the total dose of morphine required as a supplemental analgesic agent after surgery[37].
Medical uses[edit]
Opioid analgesics are commonly used to improve the patient experience when it comes to intolerable acute pain. The source of the pain sensation may result from various reasons, including chronic diseases, operations and fractures. etc. The aim of using opioids may also be providing palliative care to alleviate pain among end-stage diseases, for instance, cancer, chronic heart failure or late-stage respiratory disease.
Generally, the first-line oral drug choices available for treating moderate pain are Oxycodone 5mg, hydrocodone 5mg with paracetamol or ibuprofen, and Hydromorphone 2mg. To achieve proper pain management, the patient usually takes the pills 3 to 4 pills a day. Yet, the only IV formulation for moderate pain is Morphine IR 7.5mg[38][39].
As for severe pain, the oral drug choices are basically the same. The only difference is that the frequency increased from 3-4 pills per day to 4-6 pills per day, and IV formulation does the same[38].
The opioid choices for pain management are not limited to those mentioned above; Tramadol, tapentadol and codeine may also be used clinically in a second-line setting, depending on the concurrent medication[38].
Adverse Effects[edit]
Opioids achieve their therapeutic effect by binding to three different types of receptors: Mu ,Delta , and Kappa. When these receptors are activated, they relieve the sensation of severe pain and produce some undesired effects. Constipation, nausea or vomiting, fatigue, confusion, diuresis, respiratory depress syndrome, and headaches are the common side effects, while delayed gastric emptying, muscle cramps and hypotension are less commonly observed[40].
Opioid Misuse[edit]
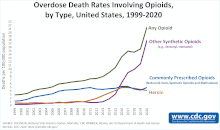
In the United States, opioid is one of the most popular medications prescribed for acute and chronic pain relief.[41] The wide use of it has led to more overdose cases reported. According to Centers for Disease Control and Prevention, opioid has lead to more than eighty thousand overdose deaths in 2021, which was 10 times of that in 1999.
Alternatives for Opioids[edit]
To lower the risk of opioid abuse, alternative non-opioid analgesics are available. These include paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs).[42] For patients with less severe conditions such as low back pain[43] or some acute trauma cases[44], NSAIDs can serve as milder painkillers, which are less likely to cause addiction or drug dependence. Another milder painkiller, paracetamol, can also be used for patients experiencing less intense pain such as knee and hip osteoarthritis. [45]
Lower Dose and Duration[edit]
If patients' pain is not well-controlled by non-opioids, short-acting opioids may be considered instead of long-acting or extended-release forms.[46] Short-acting opioids can effectively reduce the duration of drug stayed in the body, thus lowering the risk of addiction. It is also crucial to initiate the treatment with the lowest possible effective dose to minimise potential side effects. As for the prescribing duration for acute pain, three days are recommended to avoid addiction.
Patient Education[edit]
Once opioid treatment is initiated, it is essential to provide comprehensive patient education regarding the risks of overdose and strategies for prevention, as well as the disposal of opioids.[47] Moreover, patients should be reminded of the consequences of overdose, such as respiratory depression.
Some suspicious signs may indicate opioid addiction in patients, such as claiming lost or destroyed prescriptions to acquire more drugs. It is important for doctors to verify the validity of the claims and closely monitor the dosing regimen to identify signs of addiction at an early stage.
- ^ a b c Brownstein, M J (1993-06-15). "A brief history of opiates, opioid peptides, and opioid receptors". Proceedings of the National Academy of Sciences of the United States of America. 90 (12): 5391–5393. ISSN 0027-8424. PMID 8390660.
- ^ Howard-Jones, Norman (1971). "The Origins of Hypodermic Medication". Scientific American. 224 (1): 96–103. ISSN 0036-8733.
- ^ Feige, Chris; Miron, Jeffrey A. (2008-10-10). "The opium wars, opium legalization and opium consumption in China". Applied Economics Letters. 15 (12): 911–913. doi:10.1080/13504850600972295. ISSN 1350-4851.
- ^ Duarte, Danilo Freire (Feb 2005). "Opium and opioids: a brief history". Revista Brasileira de Anestesiologia. 55: 135–146. doi:10.1590/S0034-70942005000100015. ISSN 0034-7094 – via Sci Flo Brazil.
- ^ Rägo, Lembit; Santoso, Budiono (2008). Drug regulation: history, present and future. Drug benefits and risks: International textbook of clinical pharmacology.
- ^ Jannetto, Paul J; Helander, Anders; Garg, Uttam; Janis, Gregory C; Goldberger, Bruce; Ketha, Hemamalini (2019-02-01). "The Fentanyl Epidemic and Evolution of Fentanyl Analogs in the United States and the European Union". Clinical Chemistry. 65 (2): 242–253. doi:10.1373/clinchem.2017.281626. ISSN 0009-9147.
- ^ a b c Trescot, Andrea M. (2008-03-14). "Opioid Pharmacology". Pain Physician. 2s, 11 (3, 2s): S133–S153. doi:10.36076/ppj.2008/11/s133. ISSN 2150-1149.
- ^ a b Waldhoer, Maria; Bartlett, Selena E.; Whistler, Jennifer L. (2004). "Opioid receptors". Annual Review of Biochemistry. 73: 953–990. doi:10.1146/annurev.biochem.73.011303.073940. ISSN 0066-4154. PMID 15189164.
- ^ "Opioids", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012, PMID 31643200, retrieved 2024-04-01
- ^ Murphy, Patrick B.; Bechmann, Samuel; Barrett, Michael J. (2024), "Morphine", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30252371, retrieved 2024-04-01
- ^ Anderson, Ilene B; Kearney, Thomas E (Jan 2000). "Use of methadone". Western Journal of Medicine. 172 (1): 43–46. doi:10.1136/ewjm.172.1.43. ISSN 0093-0415. PMC 1070723. PMID 10695444.
- ^ "Pentazocine", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012, PMID 31643817, retrieved 2024-04-01
- ^ "Pethidine - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2024-04-01.
- ^ Pratiwi, Rimadani; Noviana, Eka; Fauziati, Rizky; Carrão, Daniel Blascke; Gandhi, Firas Adinda; Majid, Mutiara Aini; Saputri, Febrina Amelia (2021-02-04). "A Review of Analytical Methods for Codeine Determination". Molecules. 26 (4): 800. doi:10.3390/molecules26040800. ISSN 1420-3049. PMC 7913935. PMID 33557168.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Research, Center for Drug Evaluation and (2023-10-19). "Codeine Information". FDA.
- ^ Australia, Healthdirect (2023-07-12). "Fentanyl". www.healthdirect.gov.au. Retrieved 2024-04-01.
- ^ "Oxycodone (Oral Route) Description and Brand Names - Mayo Clinic". www.mayoclinic.org. Retrieved 2024-04-01.
- ^ Aronson, J. K., ed. (2016-01-01), "Remifentanil", Meyler's Side Effects of Drugs (Sixteenth Edition), Oxford: Elsevier, pp. 98–106, ISBN 978-0-444-53716-4, retrieved 2024-04-01
- ^ a b "Pharmacological Treatment | Medication Assisted Recovery". Opioids. Retrieved 2024-04-01.
- ^ a b Jamison, Robert N.; Mao, Jianren (Jul 2015). "Opioid Analgesics". Mayo Clinic Proceedings. 90 (7): 957–968. doi:10.1016/j.mayocp.2015.04.010. ISSN 0025-6196.
- ^ Gesztesi, Z.; Mootz, B. L.; White, P. F. (Nov 1999). "The use of a remifentanil infusion for hemodynamic control during intracranial surgery". Anesthesia and Analgesia. 89 (5): 1282–1287. ISSN 0003-2999. PMID 10553851.
- ^ Brunton, Laurence (2018). Brunton, Laurence L.; Hilal-Dandan, Randa; Knollmann, Björn C.; Goodman, Louis Sanford; Gilman, Alfred; Gilman, Alfred Goodman (eds.). Goodman & Gilman's The pharmacological basis of therapeutics (Thirteenth edition ed.). New York Chicago San Francisco: McGraw Hill Education (published December 5, 2017). ISBN 978-1-259-58473-2.
{{cite book}}:|edition=has extra text (help) - ^ Abuse, National Institute on Drug (2019-04-11). "How Naloxone Saves Lives in Opioid Overdose | National Institute on Drug Abuse (NIDA)". nida.nih.gov. Retrieved 2024-04-01.
- ^ James, Arul; Williams, John (May 2020). "Basic Opioid Pharmacology — An Update". British Journal of Pain. 14 (2): 115–121. doi:10.1177/2049463720911986. ISSN 2049-4637.
- ^ U.S Food and drug administration (Jan 2020). "Clinical Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry". U.S Food and drug administration. Retrieved 27 March 2024.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c U.S. Food and Drug Administration (6 May 2023). "Drug Development and Drug Interactions | Table of Substrates, Inhibitors and Inducers". U.S. Food and Drug Administration. Retrieved 27 March 2024.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Brian, R. Overholser; David, R. Foster (25 Sep 2011). "Opioid Pharmacokinetic Drug-Drug Interactions". American Journal of Managed Care. 17 (11) – via American Journal of Managed Care.
- ^ Dezfulian, Cameron; Orkin, Aaron M.; Maron, Bradley A.; Elmer, Jonathan; Girotra, Saket; Gladwin, Mark T.; Merchant, Raina M.; Panchal, Ashish R.; Perman, Sarah M.; Starks, Monique Anderson; van Diepen, Sean; Lavonas, Eric J.; On behalf of the American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Council on Clinical Cardiology (2021-04-20). "Opioid-Associated Out-of-Hospital Cardiac Arrest: Distinctive Clinical Features and Implications for Health Care and Public Responses: A Scientific Statement From the American Heart Association". Circulation. 143 (16). doi:10.1161/CIR.0000000000000958. ISSN 0009-7322.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Abuse, National Institute on Drug (2022-01-11). "Naloxone DrugFacts | National Institute on Drug Abuse (NIDA)". nida.nih.gov. Retrieved 2024-03-27.
- ^ Panchal, Ashish R.; Bartos, Jason A.; Cabañas, José G.; Donnino, Michael W.; Drennan, Ian R.; Hirsch, Karen G.; Kudenchuk, Peter J.; Kurz, Michael C.; Lavonas, Eric J.; Morley, Peter T.; O’Neil, Brian J.; Peberdy, Mary Ann; Rittenberger, Jon C.; Rodriguez, Amber J.; Sawyer, Kelly N. (2020-10-20). "Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 142 (16_suppl_2). doi:10.1161/CIR.0000000000000916. ISSN 0009-7322.
- ^ "Antihistamines present in some opioid overdoses, deaths". www.healio.com. Retrieved 2024-03-27.
- ^ Dinwiddie, Amanda T. (2022). "Notes from the Field: Antihistamine Positivity and Involvement in Drug Overdose Deaths — 44 Jurisdictions, United States, 2019–2020". MMWR. Morbidity and Mortality Weekly Report. 71. doi:10.15585/mmwr.mm7141a4. ISSN 0149-2195.
- ^ Abuse, National Institute on Drug (2022-11-07). "Benzodiazepines and Opioids | National Institute on Drug Abuse (NIDA)". nida.nih.gov. Retrieved 2024-03-27.
- ^ Nielsen, Suzanne; Lintzeris, Nicholas; Bruno, Raimondo; Campbell, Gabrielle; Larance, Briony; Hall, Wayne; Hoban, Bianca; Cohen, Milton L.; Degenhardt, Louisa (Feb 2015). "Benzodiazepine Use among Chronic Pain Patients Prescribed Opioids: Associations with Pain, Physical and Mental Health, and Health Service Utilization". Pain Medicine. 16 (2): 356–366. doi:10.1111/pme.12594. ISSN 1526-2375.
- ^ Jones, A. W.; Kugelberg, F. C.; Holmgren, A.; Ahlner, J. (2011-03-20). "Drug poisoning deaths in Sweden show a predominance of ethanol in mono-intoxications, adverse drug-alcohol interactions and poly-drug use". Forensic Science International. 206 (1–3): 43–51. doi:10.1016/j.forsciint.2010.06.015. ISSN 1872-6283. PMID 20630671.
- ^ Miranda, H F; Silva, E; Pinardi, G (2004-05-01). "Synergy between the antinociceptive effects of morphine and NSAIDs". Canadian Journal of Physiology and Pharmacology. 82 (5): 331–338. doi:10.1139/y04-027. ISSN 0008-4212.
- ^ Devlin, John W.; Skrobik, Yoanna; Gélinas, Céline; Needham, Dale M.; Slooter, Arjen J. C.; Pandharipande, Pratik P.; Watson, Paula L.; Weinhouse, Gerald L.; Nunnally, Mark E.; Rochwerg, Bram; Balas, Michele C.; van den Boogaard, Mark; Bosma, Karen J.; Brummel, Nathaniel E.; Chanques, Gerald (Sep 2018). "Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU". Critical Care Medicine. 46 (9): e825–e873. doi:10.1097/CCM.0000000000003299. ISSN 0090-3493 – via Critical care medicine.
- ^ a b c "UpToDate". www.uptodate.com. Retrieved 2024-04-08.
- ^ OPEN Prescribing Recommendations- Adult (PDF) (Report). OPEN: Overdose Prevention Engagement Network. 2024. doi:10.56137/open.000054.
- ^ "Shibboleth Authentication Request". login.eproxy.lib.hku.hk. Retrieved 2024-04-08.
- ^ Dowell, Deborah; Ragan, Kathleen R.; Jones, Christopher M.; Baldwin, Grant T.; Chou, Roger (2022-11-04). "CDC Clinical Practice Guideline for Prescribing Opioids for Pain — United States, 2022". MMWR. Recommendations and Reports. 71 (3): 1–95. doi:10.15585/mmwr.rr7103a1. ISSN 1057-5987. PMC 9639433. PMID 36327391.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Sinatra, Raymond S.; Jahr, Jonathan S.; Watkins-Pitchford, J. Michael, eds. (2010-10-14). "The Essence of Analgesia and Analgesics". Cambridge University Press. doi:10.1017/cbo9780511841378 – via Cambridge University Press.
- ^ Malik, Khalid; Nelson, Ariana (2018), "Overview of Low Back Pain Disorders", Essentials of Pain Medicine, Elsevier, pp. 193–206.e2, doi:10.1016/b978-0-323-40196-8.00024-3, ISBN 978-0-323-40196-8, retrieved 2024-04-08
- ^ Oyler, Douglas R.; Parli, Sara E.; Bernard, Andrew C.; Chang, Phillip K.; Procter, Levi D.; Harned, Michael E. (Sep 2015). "Nonopioid management of acute pain associated with trauma: Focus on pharmacologic options". Journal of Trauma and Acute Care Surgery. 79 (3): 475–483. doi:10.1097/TA.0000000000000755. ISSN 2163-0755.
- ^ Abdel Shaheed, Christina; Ferreira, Giovanni E; Dmitritchenko, Alissa; McLachlan, Andrew J; Day, Richard O; Saragiotto, Bruno; Lin, Christine; Langendyk, Vicki; Stanaway, Fiona; Latimer, Jane; Kamper, Steven; McLachlan, Hanan; Ahedi, Harbeer; Maher, Christopher G (April 2021). "The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews". Medical Journal of Australia. 214 (7): 324–331. doi:10.5694/mja2.50992. ISSN 0025-729X.
- ^ Dowell, Deborah; Ragan, Kathleen R.; Jones, Christopher M.; Baldwin, Grant T.; Chou, Roger (2022-11-04). "CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022". MMWR. Recommendations and Reports. 71 (3): 1–95. doi:10.15585/mmwr.rr7103a1. ISSN 1057-5987.
- ^ Pilling, Stephen; Strang, John; Gerada, Clare (2007-07-28). "Psychosocial interventions and opioid detoxification for drug misuse: summary of NICE guidance". BMJ. 335 (7612): 203–205. doi:10.1136/bmj.39265.639641.AD. ISSN 0959-8138.

