Tetraethoxymethane

| |
| Names | |
|---|---|
| Preferred IUPAC name
(Triethoxymethoxy)ethane | |
| Other names
Tetraethyl orthocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.985 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H20O4 | |
| Molar mass | 192.25 g·mol−1 |
| Appearance | liquid |
| Density | 0.919 |
| Boiling point | 159.5 °C (319.1 °F; 432.6 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| H226, H315, H319, H335 | |
| Related compounds | |
Other cations
|
Tetraethoxysilane |
Related compounds
|
Tetramethoxymethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetraethoxymethane is a chemical compound which is formally formed by complete ethylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarbonic acid violates the Erlenmeyer rule and is unstable in free state).
History[edit]
Tetraethoxymethane was described the first time in 1864.[1]
Synthesis[edit]
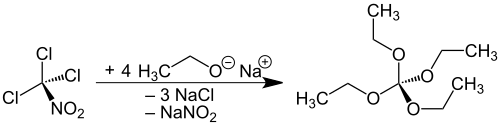
The preparation of tetraethoxymethane from the highly toxic trichloronitromethane is known in the literature[1][2][3][4] and achieves only yields of 46-49[3] to 58%:[4]
The obvious synthetic route from tetrachloromethane does not provide the desired product, as in the homologous tetramethoxymethane.[5]
Starting from the less toxic trichloroacetonitrile (compared with trichloronitromethane), higher yields can be obtained (up to 85%).[6] An alternative reaction, bypassing problematic reactants, is the reaction of dialkyltin dialkoxides with carbon disulfide at elevated temperature in an autoclave:[7]
A more recent synthesis starts directly from sodium ethoxide, tin(IV)chloride, and carbon disulfide.[8]
Properties[edit]
Tetraethoxymethane is a water-clear, aromatic or fruity smelling,[9] liquid of low-viscosity which is unstable against strong acids and strong bases.[10]
Uses[edit]
Tetraethoxymethane can be used as a solvent and for the alkylation of CH-acidic compounds (e.g. phenols and carboxylic acids). In addition, it reacts with amines, enol ethers and sulfonamides,[11] whereby spiro compounds can also be obtained. Spiro orthocarbonates (SOCs)[12] are of some industrial interest, as they are used as additives for reducing shrinkage during the polymerization of epoxides (they are used as expanding monomers).[13]
References[edit]
- ^ a b H. Bassett, Ueber das vierfach-basische kohlensaure Aethyl, Ann. 132, 54 (1864), doi:10.1002/jlac.18641320106.
- ^ H. Tieckelmann, H.W. Post, The preparation of methyl, ethyl, propyl, and butyl orthocarbonates, J. Org. Chem., 13 (2), 265–267 (1948), doi:10.1021/jo01160a014.
- ^ a b "Ethyl Orthocarbonate". Organic Syntheses. doi:10.15227/orgsyn.032.0068.
- ^ a b Europäische Patentschrift EP 0881212 B1, Production method of aminobenzene compound, Erfinder: H. Hashimoto et al., Anmelder: Takeda Chemical Industries, Ltd., veröffentlicht am 30. Oktober 2001.
- ^ R.H. De Wolfe, Carboxylic ortho acid derivatives: preparation and synthetic applications, Organic Chemistry, Vol. 14, Academic Press, Inc. New York – London, 1970, ISBN 978-0-12-214550-6.
- ^ US-Patent US 6825385, Process for the preparation of orthocarbonates, Erfinder: G. Fries, J. Kirchhoff, Anmelder: Degussa AG, erteilt am 30. November 2004.
- ^ S. Sakai et al., Reaction of Dialkyltin Dialkoxides with Carbon Disulfide at Higher Temperature. Preparation of Orthocarbonates, J. Org. Chem., 36 (9), 1176 (1971), doi:10.1021/jo00808a002.
- ^ S. Sakai et al., A new method for preparation of tetraalkyl orthocarbonates from sodium alkoxides, tetrachlorostannane, and carbon disulfide, Synthesis 1984 (3), 233–234, doi:10.1055/s-1984-30785.
- ^ J. H. Ruth, Odor Thresholds and Irritation Levels of Several Chemical Substances: A Review, Am. Ind. Hyg. Assoc. J. 47, A-142 – A-151, (1986).
- ^ Sigma-Aldrich Co., product no. {{{id}}}.
- ^ W. Kantlehner et al., Die präparative Chemie der O- und N-funktionellen Orthokohlensäure-Derivate, Synthesis, 1977, 73–90.
- ^ Vodak, David T.; Braun, Matthew; Iordanidis, Lykourgos; Plévert, Jacques; Stevens, Michael; Beck, Larry; Spence, John C. H.; O'Keeffe, Michael; Yaghi, Omar M. (2002-04-11). "One-Step Synthesis and Structure of an Oligo(spiro-orthocarbonate)". Journal of the American Chemical Society. 124 (18). American Chemical Society (ACS): 4942–4943. doi:10.1021/ja017683i. ISSN 0002-7863. PMID 11982342.
- ^ R. Acosta Ortiz et al., Novel diol spiro orthocarbonates derived from glycerol as anti-shrinkage additives for the cationic photopolymerization of epoxy monomers, Polymer International, 59(5), 680–685 (2010), doi:10.1002/pi.2755.