Talk:Properties of water/Archive 2
| This is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 | Archive 2 |
Self Ionization
Shouldn't there be a section on self-ionization of water, or at least a proper link to that page somewhere? I'd think it is a rather important property of the water molecule and is mentioned in passing in the Electrical Properties section. Harperska 22:00, 8 September 2006 (UTC)
Fahrenheit
I removed much of this because it stated "100 degrees was set at body temperature (now accepted as 98.6 degrees) and 0 degrees at the temperature at which equal parts of salt and water melt." This is incorrect (and somewhat irrelevant, body temperature being discussed in a water article like this?). It was initially based on the boiling point of water at 60 degrees, then he did a bunch of multiplication and wound up with 212. Zero degrees was colder than it got in Denmark (Fahrenheit hated negative numbers). See citation (at straightdope.com) for more information.Squad51 02:19, 19 November 2006 (UTC)
Oxane?
I've been trying to find a source for the claim that "another systematic name that has been accepted by IUPAC is oxane." All I could find, however, is a recommendation giving "oxidane" as the systematic name, saying that "the names aluminane, bismane, oxane, thiane, selenane, tellurane and polonane cannot be used since they are the names of saturated six-membered heteromonocyclic rings based on the Hantzsch-Widman system." (It further states that "the names 'azane' and 'oxidane' are only intended for use in naming derivatives of ammonia and water, respectively, by substitutive nomenclature.")
The specific table I linked to above is marked as "provisional", but the exact same recommendation can be found in other IUPAC documents, such as the Principles of Chemical Nomenclature, page 99. Given this, I believe the description of "oxane" as an accepted synonym should be corrected. —Ilmari Karonen (talk) 19:11, 15 December 2006 (UTC)
...as I have, now. —Ilmari Karonen (talk) 23:31, 15 December 2006 (UTC)
Specific heat error
Looks like the specific heats of water as vapor and water as liquid are reversed. Maybe just a labeling problem.
Fixed... I hope --Steven 04:18, 10 December 2006 (UTC)
My Primary reference is Physics for Scientists and Engineers, third edition by Raymond A. Serway. On page 566 it has the formula . This is the same as saying . In other words . On the same page, the book also lists the of gaseous at as and the at the same temperature as . On page 564 it lists the molar weight of as . Using these figures and rounding to three significant digits and at . Using the Water_(data_page) you get and at . None of the numbers for are even close to what is actually in the article. I used the numbers from Serway because, at or it is closest to the standard temperature of stated at the bottom of the table. I hope this is OK.
Commdweeb 17:20, 9 February 2007 (UTC)
Does water break up in nature?
in photosynthesis and stuff is water broken into hydrogen and oxygen. Some stuff seems to say that water is created in carb metabolism, but isn't the water just stuck in the middle of that carbohydrate and just gets released? Puddytang 22:40, 26 February 2007 (UTC)
Year of Celcius scale reversal?
This article states "The scale was reversed in 1744". The Anders_Celcius article states "The scale was reversed by Carolus Linnaeus in 1745, to how it is today". Presumably one is right and one is wrong.88.104.193.229 03:57, 13 April 2007 (UTC)
Article is mostly not about the molecule...
This article generally isn't about water the molecule, but water the macroscopic substance. Liquid water, ice, etc. Why then is the page titled Water (molecule)? Surely something like Water (substance), Water (compound) or even Water (chemical) would be more appropriate? 129.16.97.227 20:14, 6 June 2007 (UTC)
Water in Biology section is too short
Why isn't there more on water in our bodies and water in animal bodies. I don't know much about water but when I loooked up this article, I was hoping to see more information on how much of our bodies are water, why our bodies need water, what do our bodies do with the water when we drink it, etc. Can someone expand on the tiny "Water in Biology" section or advise where to look. Thanks! --Mezaco 19:03, 12 June 2007 (UTC)
- Did you look at the Water article? That one is supposed to cover those topics in more detail. This article (Water (molecule) focuses more on the chemistry. --Itub 08:43, 25 June 2007 (UTC)
Compressibility
The compressibility and the bulk modulus should be reciprocals of one another, but the values given in the article are not: therefore one of them must be wrong. I suspect that the value given for the compressibility is missing a power of ten (perhaps the units should be Mbar^(-1)?). The value for the bulk modulus is not useful, since it does not specify what conditions of temperature and pressure it is obtained at.
- I've amended that section after looking up the reference. It was only out by six orders of magnitude. ;-)
- 129.16.97.227 20:06, 6 June 2007 (UTC)
Still I think the figures in this section far out. I do not have all the right figures,but I know that at 1000 bar water is compressed bij about 4 % as compared to standard pressure. In my opinion this should mean that roughly on average compressibility is 0.04 per 1000 bar or 0.00004 bar-1 0r 4·10-5 bar-1 http://runt.ocean.washington.edu/swift/PTV-manual/node27.html (1 bar is approx 14.5 PSI) This and other links confirm this. —Preceding unsigned comment added by 83.116.231.1 (talk) 08:11, 8 October 2007 (UTC)
cleanup-combine
I have organized the article. Possible duplicate content marked as small text should be embeded into article. Task for someone with better English and with better chemistry & physics knowledge than me. Thank you for cooperation. --Snek01 (talk) 22:30, 15 December 2007 (UTC)
Symmetry
A while back I added the point group C2v to the property box on this page and linked to the page on molecular symmetry, which was subsequently removed. This is actual info' on the structure of the compound as this page is meant to convey and has previousluy been argued to be missing. I would propose that for all simple molecules (BF3, NH3, etc. ie excluding most large molecules whose only symmetry is the identity) that this info' be added. It is important data for understanding and deriving electronic configurations and bonding as well as giving info on chirality, dipoles etc. There is alot of editing needed on this page, and removal of useful facts does not help it. —Preceding unsigned comment added by Azo bob (talk • contribs) 22:03, 14 January 2008 (UTC)
- I agree 100%. 129.16.97.227 (talk) 15:46, 17 January 2008 (UTC)
Data for real water?
This data appears to all be for pure water only. The article should state this limitation clearly at the start. Having such chemical-theory data is a good start, but most of us are dealing with real water: tap water, rain water, lake water, sea water, etc. We want density etc data for real water. Where can one find data on the physical properties of real water? -69.87.199.87 (talk) 11:08, 6 June 2008 (UTC)
- Well, insofar as it differs from that of pure water, it's obviously going to depend on the source of the water in question. The biggest variable in general (excluding nonchemical issues such as suspended solids) is probably going to be salinity, for which you might be interested in our article on seawater. —Ilmari Karonen (talk) 13:44, 6 June 2008 (UTC)
- We also have an article on tapwater. —Ilmari Karonen (talk) 13:45, 6 June 2008 (UTC)
Bad phase diagram
The phase diagram shows the triple point of water at slightly over 1 kPa, about 65% above the correct figure of 611 kPa (the text is fine). --Vaughan Pratt (talk) 02:24, 17 June 2008 (UTC)
Where's the chemistry?
Section 2 is headed Physics and Chemistry of Water, but compared to the physics there's only a brief summary of the chemistry, and no systematic treatment. Not a useful article from the chemistry point of view. Furthermore, the main article Water has a section Water#Chemical and physical properties, under which it says 'Main article: Water (molecule)', yet there seems little or relationship between the chemistry content of that section and this article. Also there's no links to the article on Electrolysis of water. Consequently there's very little hard information here to counter public misconceptions such as the one that water can be a fuel for vehicles.Strayan (talk) 02:57, 31 July 2008 (UTC)
Why is it called OH, not HO?
Since my teacher at collage could not answer this question I'll ask it here: Why is it called OH in reaction formulas and not HO? I don't se the logic behind it. Surely it has historical reasons but then why is the sulfur compound is called HS? Could some please tell me more about why this is so? There has to be a reason. /curious collage student in Uppsala. —Preceding unsigned comment added by 130.243.221.26 (talk) 20:18, 17 October 2007 (UTC)
- Because, thanks to Jesse Jackson, it is not longer acceptable to label anything as a HO.
- Seriously, though, this is not a forum for discussing the topic, it is a forum for discussing the article. Please do not make forum-like posts on this page, even if you are studying collage. --Jaysweet 20:22, 17 October 2007 (UTC)
- I am serious, besides; I really do think it is older than this and as far as I know Jesse Jackson has nothing to do with chemistry. Can someone more knowledgable person please give the hisorical reasons for my question? /student in Uppsala.
- It is my contention that it doesn't really have a formal way to be written. I think that both ways of writing the chemical are acceptable. I think that OH is often written as such because it is a common functional group with the R' group being attached to the O and not the H, such as CH3COOH. Written as a stand-alone molecule it is written as H20 or H02. I think it has become somewhat arbitrary dogma, because as you should know by now, the chemical compound does not change, whether you write it one way or the other. You could also check out SMILES to see if you answer is in there. 146.244.241.204 (talk) 19:43, 28 April 2008 (UTC)niubrad
- The OH notation is due to the fact that, as the contributor above stated, hydroxyl (OH) groups are linked to other molecules by a bond to the oxygen molecule, hence appear generally in the form R-OH. R-HO would imply a bond to the hydrogen molecule, which is impossible. Adacore (talk) 05:35, 20 July 2008 (UTC)
- H2O is acceptable, HO2 (which you mentioned) is not, that's something different (hydroperoxyl). Stonemason89 (talk) 17:53, 18 August 2008 (UTC)
- I am serious, besides; I really do think it is older than this and as far as I know Jesse Jackson has nothing to do with chemistry. Can someone more knowledgable person please give the hisorical reasons for my question? /student in Uppsala.
Ligand
Can water be used as a ligand? Stonemason89 (talk) 17:49, 18 August 2008 (UTC)
Nevermind, found an answer to my own question. Added it to the article. Stonemason89 (talk) 11:36, 6 September 2008 (UTC)
Other liquids that are less dense when frozen
I am having some trouble deciding what other elements besides water will expand when frozen, according to this article. In the section called Density of water and ice, according to the text, "Water, lead, uranium, neon and silicon are some of the few materials which expand when they freeze". To me, this indicates that lead, uranium, neon and silicon are less dense in the solid state than in the liquid state.
However, if you read on in the same section, you can read that "Water shares the higher-density liquid state with only a few materials like gallium, germanium, bismuth and antimony." Now it seems that gallium, germanium, bismuth and antimony are actually the elements that are less dense as solids than as liquids. So which group of elements is actually less dense as a solid than as a liquid? Or, is it all of the aforementioned elements? If so, the section should be re-worded so that it is more clear. --Ean5533 ( View! / Talk!) 00:06, 16 February 2008 (UTC)
- Naming the sentenses (a) = "Water, lead, uranium, neon and silicon are some of the few materials which expand when they freeze" and (b) = "Water shares the higher-density liquid state with only a few materials like gallium, germanium, bismuth and antimony."
- By 3 Sep 2007 http://en.wikipedia.org/w/index.php?title=Water_(molecule)&oldid=155510615 (a) said "Water, gallium, bismuth, antimony and silicon are some of the few materials which expand when they freeze" and (b) didn't exist. The info at the links seems to support current (b) and old (a), but not current (a). At some point (a) may have been subject to sabotage/vandalism, but I really don't know. Maybe both (a) and (b) are true. David A se (talk) 23:19, 7 December 2008 (UTC)
HEAT CAPACITY ANYONE?! Thanks for heat of vaporization... tho. Alex. —Preceding unsigned comment added by 128.46.105.47 (talk) 02:00, 24 April 2008 (UTC)
ortho- and para-water
There are two kinds of water molecule which differs in orientation of spins of hydrogen atoms. It is mentioned in Spin isomers of hydrogen, and there is also a reference to the article about the separation of ortho- and para- water. I think that this should be at least mentioned here. Unfortunately my English is poor, and I'm not so strong in physics too, so I cant't do it myself. =( Para-water is valuable for nuclear magnetic resonance applications in medicine (magnetic resonance imaging). —Preceding unsigned comment added by Shcha (talk • contribs) 05:13, 1 February 2009 (UTC)
Is water actually a molecule?
Is it not true that each hydrogen is only connected to an oxygen for a fraction of a nanosecond in liquid water? certainly this is important. —Preceding unsigned comment added by 58.175.57.243 (talk) 03:48, 7 February 2009 (UTC)
Miscibility of water vapour with air
The article states that water vapour is miscible with air. Is this definitely correct? I am aware that air-overpressure autoclaves (which use a mixture of steam and air rather than pure steam as in a conventional autoclave) are fitted with powerful fans in order to keep the air and steam mixed. I understood that without these fans the air and steam separate out into distinct layers - presumably this couldn't happen if they were truly miscible in all proportions. Can someone more expert than me comment on this? —Preceding unsigned comment added by Pharmagiles (talk • contribs) 13:01, 9 February 2009 (UTC)
Official/formal name (again)
Seems we have this debate or edit-pattern often, so let's just consensus...what are the official, formal scientific names for water that are worth mentioning? That is, IUPAC-recognized or similarly WP:RS/authoritatively-citeable, not just "let's follow some systematic rules and see what one can make up", even for very famous "one"s who might do so. DMacks (talk) 21:05, 9 February 2009 (UTC)
- Dihydrous oxide is widely accepted by scientists and top-tier professors. Also, it bothers me how my Harvard professor says Dihydrous oxide is water, yet you idiots continually revert it without even looking it up. Literally, just try it. 71.115.3.186 (talk) 21:16, 9 February 2009 (UTC)
- You can read a few screens above where we did look it up and found...not what you say. DMacks (talk) 22:11, 9 February 2009 (UTC)
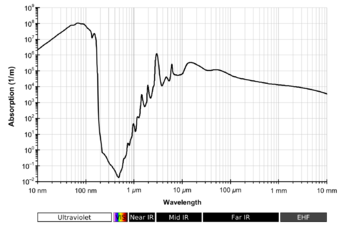
First, I think this section could be renamed something like "electromagnetic absorption" or "absorption spectra" which is more general and accurate than simply "transparency", but at the same time I do not think it is bad to keep this header simple since it would be less jargon for the non-technical readers. Does anyone else have an opinion? My vote is rename it "absorption spectra."
Second, I cleaned it up by linking it to water absorption which seems to be the main article concerning the light absorbing characteristics of water.
Third, I take issue with the line "If water were not transparent, sunlight, essential to aquatic plants, would not reach into seas and oceans." It sounds wrong to me because it implies that the absorption spectra of water has no bearing on aquatic plants' photosynthetic pigments. The fact that water and by extension the atmosphere (its absorption spectra is influenced by water vapor) is transparent in the visible spectrum is the reason why photosynthetic pigments use visible light and the near infrared wavelengths. I know it helps that the sun radiates most of its energy (or is it the most photons total?) in the visible region. The drop in absorbance around the visible spectrum also the reason why many photoreceptors operate in the visible and near-ultraviolet range as well. (Bird, insect, and bat far-purple/near-UV photoreceptors peak at 360 (bats) and 348 (bees) and can detect down to 310nm (bats), but I digress...) The one counterexample I could come up with are infrared sensors in snakes which operate between 5 and 30 μm.
Since I feel bold, I am changing it to the not-quite-ideal-but-slightly-better: Water is relatively transparent to visible light, near ultraviolet light, and far-red light, but it absorbs far ultraviolet, infrared light, and microwaves. Most photoreceptors and photosynthetic pigments utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens take advantage of water's opaqueness to microwave radiation to heat the water inside of foods.
I put in the bit about microwave ovens as a contrast demonstrating a situation where water's opacity becomes relevant. There is a lovely diagram of water's absorbance spectra at water absorption (see above) which may look nice in this article as well, but since the "transparency" section is so small, a picture may be excessive. That's all I could think of for now. User:Sifaka as 152.16.144.213 (talk) 04:01, 12 February 2009 (UTC)
O RLY?
The density of water is dependent on the temperature of the water. This is because the density is different for salt water than for fresh water.
So you're telling me that temperature is a property conferred by salinity? Interesting. Especially since it's totally wrong, and these two statements taken together are 100% non sequitur. --75.49.222.55 03:54, 9 October 2007 (UTC)
Here's another totally wrong comment I found - "Water freezes at 0 degrees celius." This is pure ignorance - we take melting points and boiling points of elements/compounds - the rule is that the temperature is always taken where the substance turns from more viscous to less viscous (more thick to less thick. I know technically ice decreases density as it gets cooler, but it is solid, thus having a higher viscosity than water). Hence, ice melts at 273k, and water can only freeze below this temperature. Alistair.
- It looks like you're taking a part of a sentence out of context, and therefore misunderstanding the whole issue being discussed in it:
- Water will freeze at 0 °C (32 °F, 273 K), however, it can be supercooled in a fluid state down to its crystal homogeneous nucleation at almost 231 K
- In fact, the whole point there seems to be exactly what you said: freezing point (unlike melting point) isn't an intrinsic property of the material. It does use a lay-language synonym for "freezing point", but avoids that technical term. And it immediately explains why the observed temperature of freezing may indeed be quite far from what the lay public often might expect (freezing being "like melting, only in reverse"). Let's reword to avoid the lay-vs-technical language confusion. Maybe this:
- The melting point of water is 0 °C (32 °F, 273 K), however, liquid water can can be supercooled well below that temperature without freezing into ice. It can remain in a fluid state down to its crystal homogeneous nucleation at almost 231 K
- DMacks (talk) 17:46, 26 February 2009 (UTC)
Sounds good. As for the original question, I think it's a mistake to use salinity to explain the temperature dependence of the density of water. Completely salt-free (fresh) water still has temperature-dependent density. Salinity and temperature both affect the density of water, but are separate effects.
There's the complication of the effect of salinity on the temperature dependence of water - I imagine two samples of water with different salinities would exhibit different temperature-density curves. This is a distraction from the fact that the sentence presented above is clumsily mixing two concepts that should only be combined when each has been explained independently.
water in it's purest state.
Can water in it's purest state be ingested without any harm to the human body??? —Preceding unsigned comment added by 74.224.232.87 (talk) 17:00, 11 January 2009 (UTC)
Life
Water is essential to all life on earth. Why? It doesn't seem to be explained anywhere. Widsith (talk) 19:38, 23 April 2009 (UTC)
(ultra-short lived) quantum effects
Do we even need this paragraph? It talks about neutron scattering, which always shows smaller effective number of protons compared to stoichiometry, and gives a couple of primary references by the same author, and a blurb from AIP with attention-grabbing caption. It's not a property of water, it's a property of hydrogen, seen on many different hydrogen-containing compounds and on molecular hydrogen itself (I peeked at the research group's home page). It was first discovered on water, but so were many other things. --Cubbi (talk) 11:08, 20 May 2009 (UTC)
- I agree with you completely and had the same doubt about inclusion when I edited it yesterday. That's why I put the emphasis on neutron scattering right up front, because it just seemed simply an over-dramatization of fact otherwise. If there isn't vast opposition to this, I support removal. Kbrose (talk) 13:21, 20 May 2009 (UTC)
- Well, not 2 years, sorry, looked at the wrong year. These guys were doing neutron Compton scattering, and saw that the scattering from the protons is "anomalously" smaller than expected. Actually, their timeline was: 1997 on water, 2003 on formvar, 2005 on molecular hydrogen 2005 abstract. Anyway, it's not about water. --Cubbi (talk) 16:26, 22 May 2009 (UTC)
Water article titles and content
The water article states: This article is about the chemical substance. For its chemical and physical properties, see water (molecule) However, this article states: This article is about the physical properties of pure water. For its distribution and importance in life, see Water. So what was the real intent? Is that article supposed to be about the chemical substance or about its distribution and importance in life? The two articles are terribly misorganized, and obviously the titles are wrong one way or another. Seems to me *this* article is about the substance. It is almost exclusively about the properties of the liquid, not about just the molecule. Why would this article be named 'Water (molecule)'? Water's properties are distinctly about the aggregation of water molecules, not a single molecule. True, the molecular properties are a basis for it all, but you can't observe condensed matter properties in a molecule. I think the main article of the topic group should be 'Water', and special subject areas should have longer titles, such as 'The molecular properties of water', 'The physical properties of water', 'Chemistry of Water', 'The importance of water for life', etc... The main article should perhaps be an overview of all topics in some short fashion, but coherent discussion not a potpourri of topics listed in arbitrary order like both article are now. Kbrose (talk) 12:46, 21 May 2009 (UTC)
- I agree, Water (molecule) is actually physical and chemical properties of water, while Water is the general article. In fact, in water, this article is references as "main" for the subsection Water#Chemical and physical properties, and that's exactly what it is. I don't know if it's a good idea to make more separate pages, it could be neat.. or it could be messy and repetitive. There are already a few on Water (disambiguation). --Cubbi (talk) 14:57, 22 May 2009 (UTC)
I have renamed the article to Water (properties) to improve this situation. Kbrose (talk) 19:38, 5 June 2009 (UTC)
I would recommend a reorganization of properties in this article according to this sequence: molecular properties, collective (gas, then condensed) matter properties, and finally chemistry. When molecular and collective properties are intrinsically related and should be discussed together, it should be a logical deduction of molecular to collective aspects. Kbrose (talk) 21:28, 5 June 2009 (UTC)
Hydrogen Hydroxide
Isn't the name hydrogen hydroxide incorrect, as that naming formula is usually reserved for ionic compounds, isn't it?Biosci01 (talk) 22:00, 20 September 2009 (UTC)
Claims of enhanced properties
I think it was heavy-handed to delete this section. I was not trying to promote these claims - just draw attention to the fact that the claims exist. Biscuittin (talk) 10:24, 17 January 2010 (UTC)
- which nevertheless draws attention to them. An encyclopedia should not digress into rumors. Materialscientist (talk) 10:28, 17 January 2010 (UTC)
Explosive Steam
I read somewhere that water, when boiled will expand around 2700 times. Does anyone know if this is the most explosively expanding chemical? 115.166.34.227 (talk) 15:34, 13 March 2010 (UTC)
- I assume this value is based on the volume of liquid compared to volume of same molar amount of gas at its boiling point. Water does have an unusually high bp for its molecular weight, so the molar volume at bp is unusually high as well (gases have approximately the same molar volume and similar expansion properties with increasing temperature). Likewise, liquid water is somewhat dense for a small-molecule liquid, so the volume of liquid below bp is rather small for a given molar amount. To find other "high expansion at boiling" materials, probably need to look for other high-bp liquids (most gas expansion per mole) that have low molecular weight (least volume of liquid). For example, rhenium has boiling point ~15.7x that of water (remembering to use absolute temperature scale, K), so the same number of moles will be ~15.7x the volume of gas. But the atomic weight is about 10x higher also, so you need 10x the mass to get 15.7x the gas volume. The density is 21x higher also (well, even if we're off by an expansion factor at high temp, it's still in that vicinity), so the we only need 1/21 the liquid volume to have the same mass. Let's see...10x mass times 1/21 liquid volume x 15.7x gas volume...rhenium has 7.5x the expansion at boiling compared to water. But you gotta get up to around 5600°C to see it happen! quick'n'dirty calculations and estimates, feel free to double-check and add corrections! DMacks (talk) 17:09, 23 March 2010 (UTC)
Why Doesn't Ice sink in 20 C Water?
According to the chart in this article, ice is denser than water hotter than 20 C. But, ice never sinks in water. It floats in hot water. What gives?
- What chart? There is a table of density which says ice will float at any temperature. Note also that ice is usually porous and has in "reality" has lower density than tabulated in ice. Materialscientist (talk) 22:23, 16 May 2010 (UTC)
- The table of density shows that water over 20 C is less dense than ice. Therefore, ice should sink in warm water. Specifically, a cubic meter of water at 20 C has a mass of 998.2071 kg, and a cubic meter of ice has a mass of 999.8395 kg. Ice is denser, and should sink. Noloop (talk) 01:15, 17 May 2010 (UTC)
- Where does your ice density originate from? Materialscientist (talk) 01:23, 17 May 2010 (UTC)
- The table of density shows that water over 20 C is less dense than ice. Therefore, ice should sink in warm water. Specifically, a cubic meter of water at 20 C has a mass of 998.2071 kg, and a cubic meter of ice has a mass of 999.8395 kg. Ice is denser, and should sink. Noloop (talk) 01:15, 17 May 2010 (UTC)
- I suspect a misunderstanding of the table here. The table of densities refers to water, even at temperatures below 0. Ice, as mentioned in the text, is about 9% less dense than water at freezing point and will thus have a density of approximately 909.97 g/l which is far less dense than water at any temperature up to boiling point (and presumably beyond). Velela Velela Talk 07:43, 17 May 2010 (UTC)
Yep, I was equating the density of water at 0 C with the denisty of ice. Thanks for clarifying. Noloop (talk) 17:29, 17 May 2010 (UTC)
Sea water conductivity and electrocution risk
sea water conductivity should be much higher than fresh water. its should be due to high salt concentration which exist at sea water. At "electrical properties" section, a misleading remark states the opposite. To be corrected! —Preceding unsigned comment added by Yoav00 (talk • contribs) 12:43, 23 August 2010 (UTC)
- What the article says is "The low electrical conductivity of water increases significantly upon solvation of a small amount of ionic material, such as hydrogen chloride or any salt." which is correct. Velella Velella Talk 22:09, 23 August 2010 (UTC)
Many chemical formulas are wrong in IE6
I added that note because many users (perhaps more than 20%) will get wrong information. When an encyclopedia gives the wrong data, I think that that is a serious problem. I have reported this problem in several places,
but, until it is fixed, I think that the note is required. Q Science (talk) 22:41, 20 December 2010 (UTC)
Is it a hydroxy?
I always wondered if water was a hydroxy. One of my chemistry teachers said no, but I'm skeptical. Whether or not it is, I think this should be added to the article so people can avoid confusion. —Preceding unsigned comment added by 75.27.126.222 (talk) 22:56, 4 January 2011 (UTC)
ref error
i appear to have accidently caused a ref error Science editor 2 (talk) 07:42, 27 February 2011 (UTC)
- I have revered your edit because it added information which is already in the article. Materialscientist (talk) 07:46, 27 February 2011 (UTC)
Oxidane -- only for parent hydride names?
The source is already cited in the section "Oxane?". In the reference table which is included in IUPAC Red Book there are two names cited for water as it is -- "dihydrogen oxide" and "water". "Oxidane" is a name for parent hydride, personally I understand it as this name should not be used for unsubstituted compound. Am I right? Please give an advice! --Esmu Igors (talk) 18:38, 5 June 2011 (UTC)
"Pure Water"
I have removed the following statement:
- Pure Water
- Water is often viewed as simply two Hydrogen molecules and one Oxygen molecule, however water is a much more dynamic creature.
and a subsequent list of the "components" of water as being H2 and friends, in some sort of equilibrium, and now it's been re-added, so let's consensus-ize. Do people really often think this? Is it true even at a microscopic-reversability level (which isn't how people "often" look at it outside of labs, but anyway...)? And should we bother explaining what we mean by these molecules if it's not even how water actually is? Also, tritium is listed (in a confusing notation, but let's deal with the concept first)...is there any non-negligible tritium content in non-enriched water? DMacks 22:22, 12 February 2007 (UTC)
It is necessary to include the presence of air in ( ambient ) water, a matter of some scientific interest since it is not well established whether the air is in molecular or bubble form.Ladyshelby (talk) 19:08, 17 February 2012 (UTC)
electromagnetic absorption spectrum
Does anybody have access to the journal Applied Optics? I'd appreciate a copy of this article to cover this important aspect in the article. Bendž|Ť 15:17, 27 August 2007 (UTC)
The electromagnetic absorption spectrum illustration should be accompanied by a complete description of how it was measured. The illustration may be useful as the range seems unique but not without details.Ladyshelby (talk) 19:08, 17 February 2012 (UTC)
Water=Isolator!?
To say that water is a good bad conductor, i.e. a good isolator of heat is wrong. Snow might be a good isolator, but it is due to the air which is contains. Water is not a good isolator. — Preceding unsigned comment added by Mhorix (talk • contribs) 15:16, 20 February 2012 (UTC)
Density
The density was listed as 1,000 g/m^3, which I changed to 1,000 kg/m^3, which is the correct density. It was reverted and called vandalism. It seems that someone has changed it back to the correct version. It also seems that it keeps getting changed and reverted. The density of water at 4 degC is 1 g/cm^3. There are 1,000,000 cubic centimeters in a cubic meter (100 cm * 100 cm * 100 cm = 1,000,000 cm^3).
1 g/cm^3 = 1 g/mL = 1,000 g/liter = 1,000,000 g/m^3 = 1,000 kg/m^3 —Preceding unsigned comment added by 75.90.169.68 (talk) 13:37, 26 March 2009 (UTC)
- The above comment is an approximation. For precise measurement refer to the article "Kilogram"
- There is a table showing density as a function of temperature. Can someone provide the formula, if there is one, for density as a function of temperature? —Preceding unsigned comment added by 155.139.3.85 (talk) 05:33, 24 April 2011 (UTC)
- Maths troubles some people, as does the idea that a cubic metre of water could weigh a metric tonne. Perhaps visualising it as a loaded pallet of bottles of Evian would help.--Elen of the Roads (talk) 09:14, 8 May 2009 (UTC)
- Any formula for the density of water as a function of temperature would be an approximation because that function is very nonlinear. It could be expressed as an experimental Taylor series, which is a form of a polynomial function, but the accuracy of that function depends on how many terms you have in that polynomial, and how accurate the coefficients of those terms are.
98.67.110.231 (talk) 23:22, 29 July 2012 (UTC)
- Any formula for the density of water as a function of temperature would be an approximation because that function is very nonlinear. It could be expressed as an experimental Taylor series, which is a form of a polynomial function, but the accuracy of that function depends on how many terms you have in that polynomial, and how accurate the coefficients of those terms are.
The notion that the following, as claimed above, is an approximation, is specious:
1.0 g/cm^3 = 1,000,000 g/m^3 = 1,000 kg/m^3 This is mathematicall exact.
Likewise, this is also mathematically exact: 1.0 g/mL = 1,000 g/liter = 1.0 kg/liter.
98.67.110.231 (talk) 23:22, 29 July 2012 (UTC)
Likewise, this is true for most practical purposes:
1.0 g/cm^3 = 1,000,000 g/m^3 = 1,000 kg/m^3 = 1.0 Mg/m^3 = 1.0 g/mL = 1.0 kg/liter
98.67.110.231 (talk) 23:09, 29 July 2012 (UTC)
Errors in the English concerning substances that expand on freezing (solidifying)
Errors in the English concerning substances that expand on freezing (solidifying):
Notice: "Other substances that expand on freezing are silicon, gallium, germanium, antimony, bismuth, plutonium and other compounds that form spacious crystal lattices with tetrahedral coordination."
silicon, gallium, germanium, antimony, bismuth, plutonium are NOT compounds. These are all chemical elements, hence "and other compounds" is specious and incorrect.
The sentence needs to state "Other substances that expand on freezing are silicon, gallium, germanium, antimony, bismuth, plutonium, and also those chemical compounds that form spacious crystal lattices with tetrahedral coordination."
How could anyone confuse chemical elements with compounds?
98.67.110.231 (talk) 23:53, 29 July 2012 (UTC)
Energy requirements of electolysis
Is it possible to quantify the energy required to elctolyse water as STP ? In particular, is it possible to scientifically allocate an energy requirement to generate the oxygen and the equivalent for the hydrogen. Knowing the energy costs of producing hydrogen as a fuel, for example, would be helpful in understanding options for non carbon fuels. My hunch is that much of the energy expended in electrolysis of water is tied up in the oxygen released rather than the hydrogen, but it is only a hunch. Velella Velella Talk 14:33, 12 April 2012 (UTC)
- Sorry, but the above is nonsense. "Allocate an energy requirement to generate the oxygen and the equivalent for the hydrogen." Nonsense. The chemical bond that connects hydrogen and oxygen exists between the two atoms, and it is shared by both of them. The energy that was relesed when that chemical bomd was formed cannot be ascribed to either the oxygen atom or to the hydrogen atom in some ratio EXCEPT if we argue from symmetry and say 50 percent to each. The amount of energy needed to break the bond is exactly the same amount, and it could be acribed 50 percent to each. The same applies to any chemical bond - for example one between carbon and chlorine.
- PLEASE: Don't any of you who do not know anything about the Law of Conservtion of Energy try to weasel some energy out of water molecules that you didn't put there. Quit having fantasies. If there was a way, then someone a lot smarter than you would have found it long ago.
- Also, this is no place to be dealing in hunches. Your hunches are all wrong anyway, and what you ought to be doing is exercising your brain by taking high school chemistry and a lot of college courses in chemistry, physics, and chemical engineering. Make no more irritating hunches!
98.67.110.231 (talk) 00:17, 30 July 2012 (UTC)
Different isotopes in water
This article did not mention oxygen-18 - even once. Oxygen-18 is a rare, heavy isotope of oxygen just like hydrogen-2 is a rare, heavy isotope of hydrogen. Hence, a water molecule with oxygen-18 in it is a form of heavy water. Even heavier would be a molecule made up of oxygen-18, hydrogen-2, and hydrogen-1, which would have a total mass number of 21. Given oxygen-16, heavy water could have a total mass number of either 19 or 20.
98.67.110.231 (talk) 00:50, 30 July 2012 (UTC)
In kJ/kg, the "kilos" cancel out, leaving the more reasonable J/g = joule per gram.
This is poor arithmetic. Why did someone have the gall to revert my simplification?
98.67.110.231 (talk) 00:50, 30 July 2012 (UTC)
Specific Heat at Standard Temperature and Pressure
For people looking to get encyclopedic information about the chemistry of water, it is very frustrating to see the specific heat at 20 centigrade, and at 30 centigrade, but not 25. I propose (and will change the article as such) so that the value for 25 centigrade is also listed. — Preceding unsigned comment added by Semitones (talk • contribs) 20:20, 1 October 2012 (UTC)
Labels on ball and stick and space filling diagrams reversed?
I think the labels on the two diagrams of the molecule may have been accidentally reversed. — Preceding unsigned comment added by 121.220.211.228 (talk) 04:01, 4 April 2013 (UTC)
5th phase of matter?
In the main water article, a liquid crystal state is mentioned. That's apparently a state which breaks the rules for solids. Also, the ice article does not mention such a state.
~ender 2013-05-15 19:26:PM MST
Interesting, that 'new section' link doesn't let you add an explanation.
- Do you have a concern, or question regarding the article? Plasmic Physics (talk) 02:48, 16 May 2013 (UTC)
Question on moving molecules
"The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds"
Isn't that just the definition of a liquid? Or do you mean to say that the atoms of hydrogen are jumping from oxygen atom to oxygen atom?
~ender 2013-05-15 19:38:PM MST
- 'No' to both questions. Hydrogen bonding is not present in all liquids. Plasmic Physics (talk) 02:42, 16 May 2013 (UTC)
- Hmm, I guess it's just saying that as a liquid, moving molecules have to break those bonds. Where does the energy to do this come from? Wouldn't that cool down the water?
- Cool down? Good, you know the conservation of energy… now try to learn what is thermodynamic equilibrium. Incnis Mrsi (talk) 05:25, 16 May 2013 (UTC)
- I think the answer to the second question is actually "yes". Self-ionization of water means some H essentially jump off one O onto another, and even though they later recombine, it's not necessarily the same H (or even the same H3O+) that is the donor. But that's only a small effect, not the point here with regards to the hydrogen bonding between water molecules. DMacks (talk) 05:50, 16 May 2013 (UTC)
hydrol
I'm no chemist, but I found a definition for hydrol (http://encyclopedia2.thefreedictionary.com/Hydrol) that suggests it's not water. Should hydrol be removed as an alternative name for water? John (talk) 16:15, 11 July 2013 (UTC)
- This dictionary lists water as the first meaning of "hydrol", and includes two other meanings including one consistent with the definition at your link. Maybe a disambiguation page for hydrol would be good. -- Ed (Edgar181) 16:25, 11 July 2013 (UTC)
- lithol = LiOH from LiH
- beryllol = HBeOH from BeH2
- boranol = H2BOH from BH3
- methanol = H3OH from CH4
- aminol = H2NOH from NH3
- oxidanol = HOOH from H2O
- fluoranol = FOH from HF
- ergo, hydrol = HOH from H2
- Plasmic Physics (talk) 22:57, 11 July 2013 (UTC)
Viscosity error
I pretty much only created an account just for this. There is a huge mystake with the viscosity of water at room temperature. It says it is 0.001 Pa.s but in actual fact it is 0.01 Pa.s. I'm not gonnna go and find the sources cause theres a million of them. If anyone must know I am currently studying a chemical engineering degree and as a member of the scientific community I believe I must point this out. — Preceding unsigned comment added by C3145524 (talk • contribs) 07:24, 9 August 2013 (UTC)
- Out of the millions here are three. 1, 2, 3. They all say its 0.001 Pa·S. — Reatlas (talk) 07:35, 9 August 2013 (UTC)
Water is NOT the universal solvent
Water is NOT the universal solvent. There is no such thing. Please review your solubility rules. AStudent 06:30, 16 July 2006 (UTC)
- A frozen block of the same substance? DMacks 02:14, 19 July 2006 (UTC)
- Nope, that wouldn't work, the liquid and solid form would be in thermodynamic equalibrium with each other. The solid form would melt (disolving the thing it was on/in) or the liquid form would freeze, becoming one with its container (thus rendering it useless as a solvent).
- You made me panic for a second! almost thought you'd answered one of my favourite rhetoric questions, like "what colour is paint?" - Jack (talk) 02:50, 19 July 2006 (UTC)
- Yeah, was meant mostly as a mu answer. But "thermodynamic equilibrium" wasn't part of the question, nor does something have to be at a uniform temperature to have reached a steady state...what if we keep slightly cooling the bottom and maybe sides of the solid and put a small heat source just above it? DMacks 05:07, 19 July 2006 (UTC)
- Could work... But as with any liquid, you couldn't stop evapouration and the vapour would condense on the cooler, corroding it a bit, then a lot, untill it eventually ceased to work. The heater would melt the block, and from then all is lost. This universal solvent; was it supposed to dissolve anything, or any amount of anything? Sounds a bit like the grey goo hypothosis to me - Jack (talk) 16:06, 19 July 2006 (UTC)
- Water is called the universal solvent because it will dissolve most substances put into it, given time, if these substances are in a manageable form. Water dissolves sugar, salt, HCl... No one is suggesting that water will eat away at each and every substance on the face of the earth. All that's being said here is that most substances will dissolve in water. Maianess 22:03, 19 November 2006 (UTC)
- Duck Tape smeared with twinkeys and peeps, cover that with dated McDonalds chicken nuggets, rhino lining, and the Bush Iraq policy. Nothing will break it down, guaranteed. 146.244.241.204 (talk) 19:53, 28 April 2008 (UTC)niubrad
Water is called the universal solvent, and of course anything we observe we do so from an anthropomorphic viewpoint. But the point is it is a well established phrase, that is commonly used. Objectively there is no such thing as a universal material at all, there are only physical laws which to best of our knowledge act universally. So in the end the physical parameters at a certain location determine what is entropically and energetically etc... favorable.—Preceding unsigned comment added by Slicky (talk • contribs)
- I agree with Maianess. Just because water dissolves very many different materials does not require that it dissolves large quantities of them. Martin Chaplin (talk) 17:07, 22 February 2008 (UTC)
- There's no need for discussion of universal solvents in an article on the water molecule (or at least, water from a chemistry perspective). It's all waffle. Water does not dissolve most things.
- If a universal solvent did exist, maybe you could contain it with an electric or magnetic field of some kind. But this is not really an issue, as no such substance exists. Fluorine is perhaps the closest you can get, but it's not so much a solvent as just very reactive.
- To contain a universal solvent, all you'd need to do is create a saturated solution of a certain crystalline solid in that solvent, then use a container made of the aforementioned solid. Nothing weird like magnetic fields or the center of the Earth... Stonemason89 (talk) 17:53, 18 August 2008 (UTC)
- All, read WP:FORUM — Preceding unsigned comment added by ATMarsden (talk • contribs) 00:02, 11 November 2008 (UTC)
Many of you are totally over-the-hill and around-the-bend concerning universal solvents.
There are such things as "figures of speech".
You do not know the difference between a literal universal solvent and a so-called "universal solvent" that is a close approximation to the literal thing, and something that is a universal solvent for all practical purposes. Water will dissolve all chemical elements, perhaps in very small percentages, and very slowly, and water will dissolve nearly all chemical compounds, given enough time and aggitation. Hence "universal solvent" is a close approximation.
In the English language, we often use terms without quotation marks as approximations to ideal qualities or quatitities. E.G. parallel lines, equilateral triangles, orthoginal lines, superconductor (which we treat as an ideal conductor), equally likely, perfectly predictable, ideal child, perfect weather.
How about perfect laws? Fortunately, we have acquired some congressmen, senators, and legislators who know that there are no such things. The concede that they simply have to do the best that they can, but there are always "unintended consquences" for all of them. Very often, it takes another round of legislation that is necessary to do away with the worst of the unintended consquences.
Why don't you just concede that there are some unintended consequences to the term "universal solvent" and DEAL WITH IT -- by the understanding that "universal solvent" is a close approximation, and NOT ARGUING ABOUT IT ANYMORE. Most of us understand this, and we just deal with it with understanding.
98.67.110.231 (talk) 22:30, 29 July 2012 (UTC)
Water does not dissolve most elements, but it does react with many. For instance, water does not dissolve sodium metal, it reacts with sodium metal to create sodium ions and hydroxide ions, along with diatomic hydrogen molecules. This process is not the same as the process of glucose molecules dissolving in water. In the case of glucose molecules dissolving in water, there is no bond breaking or bond making, no rearrangement of atoms to create new compounds. The glucose molecules simply spread apart from one another as they interact with the water molecules. By the way, if you are looking for a "universal solvent, I am guessing that one of the lighter alcohols (2-4 carbon atoms) will appreciably dissolve more substances than water will. For instance ethanol will dissolve many ionic salts slightly, but is also miscible with hexane and other nonpolar substances.74.116.154.219 (talk) 16:06, 5 September 2013 (UTC)
Acidity and Basicity of water
````Water has a pKa of 14 and a pKb of 14. The value of 15.7 was arrived at using mistaken logic. One should use the activity of water (which is assumed to be 1) rather than the concentration of water (which is 55.6 M) when calculating the value of the pka. All other pKa values given in tables of acids are calculated using the activity of water as 1, and so if you are comparing the acidity of water to the acidity of acetic acid, nitrous acid, etc., you must use the pKa of water as 14.```` — Preceding unsigned comment added by 74.116.154.219 (talk) 16:12, 5 September 2013 (UTC)
Physics and Chemistry
The Temperature and Density table is so inaccurate it's not even funny. It completely contradicts everything else the article says. Someone please fix it. —The preceding unsigned comment was added by 65.40.167.36 (talk) 19:21, 27 February 2007 (UTC).
- Could you be more specific? Or better yet, fix it yourself? Itub 10:53, 28 February 2007 (UTC)
Density
A study done at the University of Utah shows the freezing point of pure water is more like (-48 C) [2] — Preceding unsigned comment added by Skirtend (talk • contribs) 19:31, 18 September 2013 (UTC)
Optical Properties
I am not sure where it should be in the article, but I think the optical properties of water should be mentioned, since they are so observable. The index of refraction of water should probably be listed with the other properties in the table on the right.70.231.164.83 (talk) 18:30, 11 July 2008 (UTC)
Surface tension error
The surface tension of water at 20 C is 0.0728 N/m, not 7.28 N/m.
Hydric acid?
Perhaps it should be mentioned that "hydric acid" is an incorrect name, because ALL acids contain hydrogen and dissociate into H+ and some other ion? It's more like hydroxyl acid. Or you should remove "hydric acid" altogether.
some things to look up (and add?)
(I learned all of these things in a televised lecture in some college. I forget whether it was on UWTV or the Research Channel. Both are reputable enough sources.)
exclusion zone
Liquid Crystalline Water
substances of like charge are attracted to eachother in water
water absorbs energy from the sun, which powers the surface tension effect
Self-diffusion coefficient of water
I tried to add the self-diffusion coefficient of water at 25°C to the Chembox under "Properties" , but it does not appear, when I want to see it in the preview or in the page. However, in the "View History" my addition to the Chembox appears. Since I am not so experienced in contributions to the English version of Wikipedia, somebody might be helpful to add the relatively important quantity "Self-diffusion coefficient" to the Box under "Properties"!Dr. Manfred Holz (talk) 10:28, 14 October 2013 (UTC)
Semi-protected edit request on 11 December 2013
This edit request has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
you should not allow people to be able to edit like this as this makes Wikipedia a very unreliable source therefore i will not be donating today thank you for your time reading this your good brother water
80.2.123.102 (talk) 21:55, 11 December 2013 (UTC)
basic rules of chemical nomenclature
Where does the sentence "In keeping with the basic rules of chemical nomenclature, water would have a systematic name of dihydrogen monoxide" come from? The reference given (Leigh, pp. 27–28) does not say such a thing. The systematic name should rather be hydrogen oxide. The sentence I just quoted misleads the reader into thinking that "dihydrogen monoxide" follows any accepted nomenclature.
Until this is clarified I have added the [where?] tag.
Benzocaine (talk) 21:56, 10 March 2014 (UTC)
New science - melting point UNKNOWN ! Pure water is fluid at -17C !!!
Believe it or not, but a Swedish TV-programme, showed that absolutely pure water (wich is far from destilled water) doesn't freeze even at -17 C !!! By "absolutely pure" is physically ment H2O molecules only. Not a trace of any ions. Its freezing point is little below -17, but the scientists were not certain that the water was absolutely pure. As soon as objects made of plasic, metal or wood is put down in the pure water with a temperature of -17 (but still very fluid), ice was forming around the objects. Boeing720 (talk) 17:43, 24 May 2014 (UTC)
Forgot, nothing was said about the preassure. But the focus was entirelly on the pureness of water. If a different air preassure was used, then the whole laboratory must be preassurized. (or depreassurized) Boeing720 (talk) 17:50, 24 May 2014 (UTC)
Here is a link that goes even further (down) http://news.discovery.com/earth/coldest-water-can-get-before-freezing-111123.htm Boeing720 (talk) 17:53, 24 May 2014 (UTC)
- This is not about purity, but about supercooling, which is not thermodynamic cooling. Materialscientist (talk) 22:59, 24 May 2014 (UTC)
Please read this instead http://unews.utah.edu/news_releases/supercool/ - do You still say that this has nothing to do with water and purity ? "4000 molecules" I cannot help thinking they all are common H2O's ? Why revert my entire edit, I was about to re-formulate my text. (the part about Celsius). Of course -48C/-55F is "supercool" in a sence. But please read the article, it explain why wavy water doesn't freeze. The more pure the lower the freezing point gets, as I understand it. Its of value also for higher temperatures aswell. And like I wrote earlier today, a glass of fluid but "pure" water had a temperature of -17 C. They just stated that it was not enough to use destilled water, but it seems clear to me that the new science is interesting if nothing else. Boeing720 (talk) 23:20, 24 May 2014 (UTC)
- Of course, purity matters in all phenomena, but the key here is supercooling. Materialscientist (talk) 23:25, 24 May 2014 (UTC)
OK, I guess You know much more about thiese issues than me (given Your alias, no offence ment), but even if it was a long time ago now, I have in Swedish "Jag har också trasslat in mig i Mollierdiagrammet" - it's a bit disambigous but means "I have also been captured in the Mollier diagram" (there was a picture illustrating its complexity aswell). So I'm no total fool about water, ice, steam etc.
- And "Supercooled liquid water must become ice at minus 55 F not just because of the extreme cold, but because the molecular structure of water changes physically to form tetrahedron shapes, with each water molecule loosely bonded to four others, according to the new study by chemists Valeria Molinero and Emily Moore" and "Yet in very pure water, “the only way you can form a nucleus is by spontaneously changing the structure of the liquid,” she adds." isn't enough to be of encyclopedic interest ? Somehow ? Perhaps my contribution was taken from the wrong end, so to speak. (Concerning purity) But I cannot agree to Your statement that this article is about the subject of supercooling, but sooner about of water in its physical sence. The issue isn't how to cool down the water, and a temperature of -48C/-55F are not that hard to obtain and do occur in nature (Antarktis , Sibiria). But here is explained why it cannot be liquid at even lower temperatures than that. An aboslute freezing point, if You like. And down to that temperature, water can obviously be fluid. And often are, in rivers for instance. And in sea water if we fully disreguard purity. I leave it to You though, but I think something ought to be written about it. Cheers Boeing720 (talk) 23:56, 24 May 2014 (UTC)
Calculation of the Ka value
For example:
Ka(H2O) = [OH−
]×[H+
]÷[H2O] = (Density(OH−
)÷Molar mass(OH−
))×(Density(H+
)÷Molar mass(H+
))÷(Density(H2O)÷Molar mass(H2O)) ≈ (?g/L÷17.01g/mol)×(?g/L÷1.01g/mol)÷(999.97g/L÷18.01g/mol) ≈ ?mol/L×?mol/L÷55.52mol/L
But how to get the Density(OH−
) and Density(H+
)? Or is it possible to get the [OH−
] and [H+
] directly?
Thanks. 123.119.16.126 (talk) 13:28, 27 May 2014 (UTC)
- User:Dirac66 answered at Talk:Acid dissociation constant. DMacks (talk) 18:51, 28 May 2014 (UTC)
NFPA-704 icon shows zero reactivity for water
The NFPA-704 shows toxicity in blue (0, correct), flammability in red (0, correct) and reactivity yellow (0,-incurrect-). Water is one of the more reactive chemicals that is why electronics such as cellphones cannot be in contact with water at all as the metals inside get corroded, wood gets rotten faster and it reacts with many other chemicals. So, I'd guess set the yellow box to a value of 2 out of 4. S k a t e b i k e r (talk) 07:33, 25 October 2014 (UTC)
hazard -- avalanche
I have added "avalanche" to the hazards of water -- as snow. Avalanches strike with little warning, and people caught in them are trapped so that they can easily die of suffocation or hypothermia. To be sure, avalanches do not happen on flat terrain, but they are common enough to take a toll of lives.
Snow in an avalanche sets quickly and firmly.Pbrower2a (talk) 08:11, 9 January 2015 (UTC)
bogus graph 1/4 way down, rhs ?
this graph -
http://en.wikipedia.org/wiki/Properties_of_water#mediaviewer/File:Density_of_ice_and_water_(en).svg
1/4 way down, rhs, makes no sense; the small curve fits with the data in the table but what is the large curve ? no sign of any explanation for sudden drop of density @ 0 celsius, below this the curve doesn't fit the data in the table.
— Preceding unsigned comment added by 92.238.63.233 (talk) 02:23, 13 February 2015 (UTC)
- The graph reads "Density of ice and water" and combines water to the right and ice to the left. Ice properties are described in its own article, not here. Materialscientist (talk) 03:18, 13 February 2015 (UTC)
See Wikipedia:Miscellany for deletion/Draft:Liquid crystal water.
I think editors interested in this topic would be appreciated if they could give opinions on whether this is topic of any worth. Specifically, I think the test is whether this topic, Liquid crystal water, would be worth paragraphs of coverage at this or another mainspace article. --SmokeyJoe (talk) 23:50, 13 May 2015 (UTC)
- The MFD is still open, who/where do we nudge to get it settled? Roger (Dodger67) (talk) 17:54, 13 June 2015 (UTC)
A Question on the Density of Ice
The book "Chilled" by Tom Jackson indicates that most of the expansion that occurs when water changes to ice is due to dissolved gas in the water (p. 87), and that liquid water would expand by only a few percent when changing to ice if it were boiled first to remove the dissolved gases. Is this true? Since this article focuses on pure water (i.e., without any dissolved gases), should this be in the article? Psalm 119:105 (talk) 12:06, 12 October 2015 (UTC)
Errors
Molar mass of water
- The Table claims that the molar mass of water is "18.01528(33)". This is wrong. Oxygen has an atomic mass of 15.999(4), H is 1.008(1). If I am able to count, those both are THREE significant digits to the right of the decimal point. If you don't understand the problem reporting 5 places when it just isn't possible to know more than 3, then you shouldn't be editing this. (I am not saying that, for a particular sample of water, 5 (or more) places aren't MEASURABLE. I am saying reporting that value is at best misleading (since it can ONLY refer to a specific sample or set of samples) and so is technically WRONG (it isn't "the value" for an arbitrary sample).) Additionally, a citation should be provided for this value.Abitslow (talk) 14:08, 24 February 2015 (UTC)
- There also reads: Percentage of elements in water by mass: 11.1% hydrogen, 88.9% oxygen.
- That is true, if calculated with H = 1 and O = 16. However, if the more accurate values are used, it is 11.2% hydrogen, 88,8% oxygen. 212.50.203.198 (talk) 14:48, 7 April 2015 (UTC)
- There also reads: Percentage of elements in water by mass: 11.1% hydrogen, 88.9% oxygen.
Partial vapor pressure of triple point of water: 611.73 Pa or 611.657 Pa?
The value 611.73 Pa is cited here as well as in all related artciles in Wikipedia. But the source is not provided! The most recent reference which I could find on this, Murphy and Koop (QJRMS, 2005) gives 611.657 +/- 0.01 Pa and this is also the value used by the International Association for the Properties of Water and Steam (Wagner et al., J.Phys.Chem.Ref.Data,1994). Unless the value 611.73Pa can be referenced properly, I would recommend to use the internationally recommended value. Simon Chabrillat (talk) 10:51, 23 February 2016 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified one external link on Properties of water. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Corrected formatting/usage for http://www.biophysics.org/education/parsegian.pdf
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—cyberbot IITalk to my owner:Online 13:30, 5 May 2016 (UTC)
Semi-protected edit request on 8 November 2016
This edit request to Properties of water has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
You could also die from drowning in water
2601:44:8700:36FC:1DAB:7038:90F9:45AB (talk) 13:24, 8 November 2016 (UTC)
- True Which is why drowning appears at the top of the Hazards list, in the box on the right hand side - Arjayay (talk) 13:35, 8 November 2016 (UTC)
Semi-protected edit request on 12 January 2017
This edit request to Properties of water has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
Correct first sentence to state that water is a liquid at room temperature. Blackwatch12 (talk) 00:58, 12 January 2017 (UTC)
- Seems it says that now: "that is at room temperature a tasteless and odorless liquid" 'tho' perhaps that wording is a bit stilted ... hmm. Vsmith (talk) 01:33, 12 January 2017 (UTC)
Specific vs Molar Heat Capacity
The intensive heat capacity value in the info box is per mole (Molar Heat Capacity) but labeled "Specific Heat Capacity", which is per mass. Is there a reason for this? Thelbert (talk) 21:49, 16 December 2015 (UTC)
I am sorry, but I suppose you must have done an error in normal temperature water density - "Liquid: 999.9720 kg/m3" should be "Liquid: 997.20 kg/m3" instead. Let us look at https://water.usgs.gov/edu/density.html . I went through chemical high school, I remember there was a "room" to argue that "water density is not exactly 1 (g/ml)". If it were by just 0.003%, no one would be seriously complaining, but 0.3% is something you can distinguish by normal balances. — Preceding unsigned comment added by 1x2trfn (talk • contribs) 21:57, 16 February 2017 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified 4 external links on Properties of water. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20110726171925/http://old.iupac.org/publications/books/principles/principles_of_nomenclature.pdf to http://old.iupac.org/publications/books/principles/principles_of_nomenclature.pdf
- Added archive https://web.archive.org/web/20110726171925/http://old.iupac.org/publications/books/principles/principles_of_nomenclature.pdf to http://old.iupac.org/publications/books/principles/principles_of_nomenclature.pdf
- Added archive https://web.archive.org/web/20110726171925/http://old.iupac.org/publications/books/principles/principles_of_nomenclature.pdf to http://old.iupac.org/publications/books/principles/principles_of_nomenclature.pdf
- Added archive https://web.archive.org/web/20080309145159/http://www.iapws.org/relguide/IAPWS95.pdf to http://www.iapws.org/relguide/IAPWS95.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 16:29, 22 May 2017 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified one external link on Properties of water. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20111113102454/http://www.asprs.org/a/publications/pers/2000journal/february/2000_feb_155-161.pdf to http://www.asprs.org/a/publications/pers/2000journal/february/2000_feb_155-161.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 02:01, 24 May 2017 (UTC)
Solubility
I've just reverted a good-faith edit by Weneedwikipedia to the solubility_other parameter in Template:Chembox, intending to move Weneedwikipedia's new content to solvent_other, but the template does not have this parameter. Should we record the fact that water is an excellent solvent in the infobox, or is it sufficient to record the fact later in the running text? Dbfirs 11:34, 29 May 2017 (UTC)


















