Talk:Nitrous oxide/Archive 1
| This is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 | Archive 2 |
asphyxiation risk ? Deaths have occurred from asphyxiation
Stronger statements regarding asphyxiation is needed. Years ago I read a newspaper article of a tank of N20 that was stolen from Indiana University School of Dentistry, shortly later a paramedic and his wife were found dead in their home, having inhaled the pure gas without air being mixed with it. — Preceding unsigned comment added by 172.77.225.24 (talk) 10:56, 20 November 2017 (UTC)
Recreational use
Nitrous Oxide has a HUGE following in Los Angeles. Most house parties in the area usually have Nitrous Oxide tanks. They sell the gas (in balloons). It is getting out of control. The page doesn't really talk about the recent surge in 'Nosing' —Preceding unsigned comment added by 98.148.209.177 (talk) 06:02, 7 June 2010 (UTC)
"At a recent rock festival..."
The word "recent" looks odd in an encyclopedia article. — Preceding unsigned comment added by 2601:588:4200:1C59:ED6F:4ABF:E895:926C (talk) 14:44, 12 August 2015 (UTC)
I believe it is a quote (containing the word "recent"), so if the quote is to be kept correct then the word "recent" should remain. Agreed that if the word was used in the non-quoted text then it would be inappropriate due to the lack of temporal specificity of such an encyclopedic entry. — Preceding unsigned comment added by 73.230.28.15 (talk) 01:17, 16 December 2016 (UTC)
N2O As a Gasotransmitter
There is an orphan article by the name of Gasotransmitters that says that Nitrous Oxide may be a gasotransmitter, and goes on to talk about how nitrous has a direct effect on neurotransmitters. That article should be linked somewhere in this page, but not personally being a chemist or professional on Gasotranmitters or Nitrous Oxide, I don't know how to fit it in. To help better connect the orphan to the rest of Wikipedia, someone should add the Gasotransmitters link somewhere on this article.
- I don't think nitrous oxide is not a gasotransmitter (for those of you who don't know what that it is, it is a gas that is present in the human body and probably other animals that has actions as a neurotransmitter, it is pretty interesting as neurotransmitters are usually chemicals as opposed to gases), did you confuse NO as nitrous oxide, NO is in fact nitric oxide, I believe it dilates blood vessels and this is the gasotransmitter that Viagra works on to increase (increased nitric oxide = increased blood flow + dropped blood pressure = easier erection and easier time for the heart to pump. Viagra works kind of like butyl nitrite or the other nitrites in fact.) I don't think nitrous oxide is a gasotransmitter for the simple reason there are already chemicals that regulate NMDA function, and that's the only purpose I could see nitrous oxide having. Sincerely and truly yours, C6541 (T↔C) at 06:13, 24 December 2008 (UTC)
Citation error?
The following quote of Priestley seems to refer to nitrous oxide in this article, whereas in the article about Priestley, the same quote is said to refer to oxigen:
"I have now discovered an air five or six times as good as common air... nothing I ever did has surprised me more, or is more satisfactory."
According to John Gribbins (Science: A History) the remark must have been about oxygen, not about nitrous dioxide. Watasenia 14:21, 22 May 2007 (UTC)
- Shall we delete it? NCdave 08:59, 13 September 2007 (UTC)
I fixed several spelling and grammatical errors. I don't think I need to discuss this beyond mentioning it here. 121.90.68.199 23:47, 13 October 2007 (UTC)
arrow notation
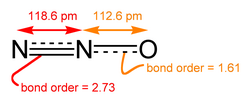
The image shows the two resonance forms of nitrous oxide as being in equilibrium with one another, which is not correct. The molecule is actually some superposition of the two resonance structures shown, which is demonstrated by using a doubleheaded arrow instead of an equilibrium arrow. I've known inorganic chemists to be more relaxed about this than organic chemists, but it is still not correct as an equilibrium arrow implies that there is some mixed population that is rapidly converting from one type to another which is completely incorrect according to our understang of quantum mechanics. Further, there isn't even any reason to believe that the state would tend to collapse into one of these states, i.e. that either is an eigenstate.
- Sorry, my mistake. Thanks for highlighting the error so eloquently. Made the image last night when tired. Fixed now.
- Ben 22:45, 8 August 2006 (UTC).
PLEASE be aware of the spam links coming from poweredbynitrous some new commercial link spam--Edited By a Professor of Life 23:26, 6 October 2006 (UTC)
spectroscopy
It would be nice to have a plot of the absorbtion spectrum. Why is this a greenhouse gas? Which wavelengths does it absorb most strongly? Due to which excitations in the molecular structure? (this unsigned comment was added on 24 October 2005 by 67.191.196.238)
It would be a help if someone could tell how the quantities of N2O in the gaseous mixture are measured. What would be the extent of uncertainty in the measurement? (this unsigned comment was added on 28 October 2005 by 59.176.20.58)
Bonding in nitrous oxide
How am I to picture the structure of this molecule? Oxygen wants to acquire two electrons, and nitrogen wants to acquire three or to shed five. So how to they come to an agreement? AxelBoldt 16:27 Nov 25, 2002 (UTC)
this is just a guess, but I'd say this: The one O bonds to both N, so it's happy :-). The angle between two bonds from O is about 120 deg (I think -- check the article on oxygen); that puts the two N close enough to form a double covalent bond between them. So each N has three bonds: one to O and a double to the other N. All present and correct. -- Tarquin 17:51 Nov 25, 2002 (UTC)
Yup, makes sense. I hope nature is smart enough to have figured that one out in the same way that you did :-)
Actually, http://www.chm.bris.ac.uk/motm/n2o/n2ov.htm claims that the the three molecules are chained, with the first N double bounded to the second which in turn is double bounded to the oxygen. AxelBoldt 16:23 Nov 26, 2002 (UTC)
As the image shows, there's a dissociated electron which flits around the molecule meaning the molecule is constantly polarised. -- ThomasWinwood 14:25, 16 Dec 2004 (UTC)
Nitrous oxide is a linear molecule, NNO. Yes, the bonding is difficult to describe with simpler models, although the two canonical forms shown in the article give an idea. There is no "dissociated electron"—nitrous oxide has an even number electrons (30 to be precise)—but there is a delocalised electron pair which "belongs" to all three atoms. Physchim62 15:13, 27 May 2005 (UTC)
- I've just been trying to make sense of it. The first thing I made out was that the molecule flips between two states. Then one comes up with (numbers denote number of outer electrons):
N(5) N (5) O (6) N(8) ≡ N (9) − O (7) covalent bonds N(8) ≡ N+(8) − O-(8) N gives O an electron N (5) N (5) O(6) N (7) = N (9) = O(8) covalent bonds N-(8) = N+(8) = O(8) N gives N an electron
- So effectively, there are both ionic and covalent bonds involved. But from what you say I make out that it's actually something between the two, a bit like the bonds between the carbon atoms in benzene are somewhere between single and double because of delocalised electrons. Looking at the bonds common to both, we get
N(5) N(5) O(6) N(7) = N(8) − O(7)
- What happens then – do two of the middle N's electrons just circle around the whole molecule? Or is there really a three-atom version of a covalent bond involved? — Smjg (talk) 12:10, 20 March 2012 (UTC)
Nomenclature of article title
I will probably move this page to Dinitrogen Oxide as that is its name. Nitrous oxide is just a comman name(make a redirect). If any has any reasons as to why I shouldn't speak up. -- Tardigrade 17:43, 9 May 2005 (UTC)
DO NOT MOVE THIS PAGE, It is reffered to and known as Nitrous Oxide N20
- Object. Nobody uses "dinitrogen oxide", even if you fix your proposed improper capitalization. Google: nearly 1000:1 nitrous oxide. Over 5000:1 if you throw out those which use both. Gene Nygaard 18:22, 9 May 2005 (UTC)
- Capitalization aside, dintiroGeN oxide is the "proper" name and as an encylopedia it should be acurate. Note that the article already says "name: Dinitrogen oxide". Is there a standard for nomenclature we should adhere to? Tardigrade 00:59, 10 May 2005 (UTC)cont. for example Baking soda redirects to Sodium bicarbonate.
- Double strong object!!
- The proposed article title is contrary to Wikipedia:Naming conventions (chemistry).
- Dinitrogen oxide is ambiguous as a name. IUPAC will provide you with dinitrogen monoxide or nitrogen(I) oxide—note that IUPAC is not a unique naming system—and these synonyms should probably be referred to in the article.
- Physchim62 15:13, 27 May 2005 (UTC)
object. more on chem naming guidelines which do state that the common name should be used as the name of the article. --Heah (talk) 17:19, 27 May 2005 (UTC)
Agree and object. How about "Nitrous oxide, formally known as dinitrogen oxide or . . ." (see my proposal to have this apply on all articles here) Twilight Realm 21:06, 12 October 2005 (UTC)
STRONGLY OBJECT this is a mistake and not 100% accurate do not move or touch this page
I think title should be kept as Nitrous Oxide, as that is the name to which it is most well known (500x as many google hits for example). However, I do think the 'name' entry in the inorganic table should include all three names. Is there specific policy on that? Jens Nielsen 20:37, 27 January 2006 (UTC)
I also object, chemistry textbooks routinely refer to this compound as nitrous oxide. If you must, include the proper chemical name in the title as well, but don't remove "nitrous oxide."
Broken link
Don't know how to fix it but...the "Hazardous Chemical Database" link in the table under safety is broken. Poseidon^3 01:50, 24 May 2005 (UTC)
- Fixed now. Physchim62 18:51, 27 May 2005 (UTC)
Laughing gas
Yes, I know that the purpose of Wikipedia is to tell facts, but do we really need to tell people that they can obtain laughing gas from whipped cream? It's a great way to increase drug use, telling anyone who wants to know an easy way to obtain the illegal substance. Guess who the only people are who would use that information? Twilight Realm 21:40, 12 October 2005 (UTC)
- We tell people how to make a Teller-Ulam device. The fact that nitrous oxide is used in whipped cream and other food applications is hardly a secret. Also, it's a little bit biased to call it an illegal substance; for instance, here in NC, USA, there are plenty of legal uses, and posession is not inherently illegal. My understaning is that is true for most of the world. There are also plenty of places where inhaling N2O for recreational purposes is legal. Evand 18:12, 13 October 2005 (UTC)
- Getting it out of whipped cream in sufficient quantities to have an effect would be quite a challenge. I think it is important to point out that nitrous oxide has legitimate uses, more so than to dwell on its actual or pending criminalisation in a minority of US states. Physchim62 17:58, 14 October 2005 (UTC)
- Contrary to Physchim62 the inhaltion of one can is all that is necessary to induce the intoxication associated with n20 use. For a deeper and longer intoxication "whippets" and a cracker and a ballon will be necessary. You can get the crackers whippets and ballons legally at head/tobacco shops. (this unsigned comment was added at 20:25, 22 February 2006 by 69.160.64.222)
- Ingestion of whipped cream is not enough to induce any serious effects from N2O (canned whipped cream would otherwise hardly be authorised under food safety regulations I imagine), but inhalation of the gas from an empty whipped cream dispenser (i.e. without cream), e.g. [1] is. It puts users in a rather inebriated state, as I could observe at one party. The effect lasts much shorter than alcohol though. Repeated N2O use can damage your nervous system, as was observed with - I kid you not - abusing dentists.--83.79.171.243 (talk) 18:35, 16 January 2009 (UTC)
Hippie Crack
This article formerly had this wording: due to some usage similarity to "crack" cocaine, it has come to be known colloquially as "hippie crack".
Although it is known as hippie crack, I don't know what 'usage similarity' means or how the way one uses crack could be similar to the way one uses cocaine. For example, one smokes crack whereas one inhales nitrous via balloon, inhalation mask or whip cream canister. However, my understanding has always been that H-crack refers to the drug's addiction potential (psychological rather than physical) . I believe some form of the language I have introduced instead will be more accurate.
Possibly this article could use more info on the history and current recreational uses of this drug, maybe in the form of an article fork. Thoughts? Kit O'Connell (Todfox: user / talk / contribs) 19:12, 23 January 2006 (UTC)
- I was under the impression that the comparison to crack came from the quick onset and dissipation of the effects of nitrous, and the "hippie" part came from comparing the stereotypical nitrous user to the stereotypical crack cocaine user. —alxndr (t) 22:54, 23 January 2006 (UTC)
- Hmm, that's interesting. Its so hard to tell with slang. Maybe we can find some citations for one or the other or both. I agree about the hippie part of course but have heard a different reason for the crack part as I explained. It is definitely possible both are true. Maybe there is a good way to further adjust the wording. Kit O'Connell (Todfox: user / talk / contribs) 07:20, 24 January 2006 (UTC)
OK, a new definition has been placed in the article -- Hippie Crack now reflects both the purported psychological addictiveness of the substance as well as the short duration of effects -- both my definition and Alxandr's. How does this look now? Kit O'Connell (Todfox: user / talk / contribs) 21:30, 25 January 2006 (UTC)
- Nitrous oxide is no where near the addictive potential level of cocaine and especially smoked freebased cocaine. Hippie crack in fact refers only to the fact that it is quick onset, moderately intense, short peak, and quick comedown and even possibly because they are both inhaled, the hippie prefix refers to either the stereotypical user or the fact it is readily available, cheap and sometimes seen as dirty as an inhalant to uneducated people. N20 can in select individuals can lead to a somewhat psychologically dependent state, but as with other NMDA antagonists collectively referred to as the dissociatives it is typically non-addictive and sometimes extremely unpleasant. Sincerely and truly yours, C6541 (T↔C) at 06:02, 24 December 2008 (UTC)
- realted to this, but not in hope of improving the article, but merely out of curiosity: Am I right in assuming that crack cocaine is far more hazardous to your health than laughing gas? I mean, in case you were forced at gunpoint to do one of them, laughing gas would by far be the preferable, even the addiction excluded, looking merely on the damage done by one single occurence of use? 62.107.24.213 (talk) 11:18, 11 May 2009 (UTC)
less pharmacology/recreational focus please
The article seems to me rather disproportionate in its focus on recreational and pharmacological aspects. I suggest rearranging the sections a bit, as N2O is interesting for many other reasons and professions. For 'legality' I can't see for whom it is relevant enough to include here except the recreational users, so I suggest to relegate it to a subsection of the recreational use section. Neuropharmacology is also mostly relevant to the medical part (and in part for the recreational), and how about including this section under the medical uses? Jens Nielsen 20:31, 27 January 2006 (UTC)
I was looking for information on the synthesis of nitrous oxide (if anyone can help with that, please comment) and I found this article and another on studycrime.com and they're...pretty darn similar. Either the origins of this article come from the one at studycrime, or someone there copied this one over. Either way I felt like this should be brought to the community, so here's the link
http://www.studycrime.com/Schedule-VI-Controlled-Substances/Nitrous_oxide.php
I believe industrial manufacture is by controlled decomposition of ammonium nitrate (NH4NO3 -> 2H2O + N2O). I have a (hardcopy) reference paper on AN decomposition that includes more details of the reaction that I can find at some point (iirc, it has to be done at modest temperature and reduced pressure, to speed the reaction, ensure purity, and prevent explosion). Evand 19:18, 8 February 2006 (UTC)
- I believe you're correct, but alas I don't have a citable reference on me for the moment. As a small scale lab demonstration, this works: you can show that nitrous oxide relights a glowing splint as does oxygen. Physchim62 (talk) 08:00, 23 February 2006 (UTC)
Although slightly off topic, I thought it might add to the content of this article if N2O's use as a body building supplement was mentioned. This has been a recent trend in the body building community, supposedly for it's effect on the immune system, which leads to larger, faster gains in muscle mass.
- No, you are thinking of NO (nitric oxide). Nitrous oxide has no use in bodybuilding. Vorpal22 14:24, 2 July 2007 (UTC)
PLEASE HELP ME
CAN ANYONE PLEASE TELL ME THE CLASS OF DRUG THAT NITROUS OXIDE IS?
- Nitrous is regualted by the FDA but it is not scheduled nationally. In most areas nitrous possesion and sale is legal, however misuse is illegal, for more information www.erowid.org. (this unsigned comment was added at 20:28, 22 February 2006 by 69.160.64.222)
On the off chance that you're talking about pharmaceutical class, nitrous oxide is a general anesthetic, ATC code NO1AX13. Physchim62 (talk) 23:40, 22 February 2006 (UTC)
- For recreational use, it is often classified as a dissociative. --Muugokszhiion 06:23, 23 February 2006 (UTC)
Nitrous oxide laws
Can anyone tell me what the laws againt nitrous oxide in australia,switzerland,england and USA (calafornia)
email:raven_blackness@hotmail.com please reply it a school assigment
- Perhaps this link can help. --Muugokszhiion 18:08, 2 May 2006 (UTC)
Dangers of heating ammonium nitrate
According to the Wikipedia article on ammonium nitrate: Ammonium nitrate is an explosive in its purest form although it is an unusually insensitive one. Explosive properties become much more evident at elevated temperatures. When ammonium nitrate is fused and "boiled" to generate nitrous oxide it has been claimed to be as sensitive as dynamite at the ~240 C operating temperature. How badly this exothermic reaction can run away and reach detonation velocities (without proper temperature controls) has been demonstrated several times, most notably at the Ohio Chemical plant in Montreal in 1966.
This article goes on to include a list of catastrophic explosions involving the compound, including the Texas City disaster of 1947 (the worst industrial accident in US history, with at least 700 dead or missing.) Another case on a much smaller scale worth including here is of a botched attempt to make anhydrous ammonia for methamphetamine manufacture. [2] There was no need to heat the ammonium nitrate at all in this case; adding it to a sodium hydroxide solution would have generated its own heat and passed the desired anhydrous ammonia gas through the outtake hosing.
I'd hate to see people be killed or maimed in order to make their own intoxicants, let alone by being caught up in someone else's such actions.---BDH
Exothermic or not?
There seems to be a contradiction.
":NH4NO3 -> N2O + 2H2O + 58.6 kJ:
The addition of various phosphates favors formation of a purer gas. This reaction occurs at around 240°C, a temperature where ammonium nitrate is a moderately sensitive explosive and a very powerful oxidizer (perhaps on the order of fuming nitric acid). At temperatures much above 240°C the exothermic reaction may run away" Guinnog 06:17, 2 May 2006 (UTC)
- What contradition are you referring to? NH4NO3 is highly explosive, and explodes (in an exothermic reaction, of course) with enough input energy (temperature). This source claims the reaction enthalpy is -36 KJ/mol, by the way. --Muugokszhiion 18:06, 2 May 2006 (UTC)
- The contradiction is between the positive delta H and the exothermic claim. One is wrong. Guinnog 18:30, 2 May 2006 (UTC)
- Huh? The reaction shown lists 58.6 kJ energy as a product, ie the energy is released as part of the decomposition. I don't see a ΔH in there anywhere. I have a section of Nitric Acid and Fertilizer Nitrates by Shah and Roberts (hardcopy; I don't know if it's online anywhere) that lists ΔH = -8.8 kcal/mol (aka 36.8 kJ/mol released).
- I see. How confusing. I'm used to the convention that a +ve ΔH denotes an endothermic reaction. Guinnog 23:55, 3 May 2006 (UTC)
- A positive ∆H DOES denote an endothermic reaction, but an alternative notation is to write the ∆H as a positive number on one side of the reaction or another... on the reactants side for an endothermic reaction or on the products side for an exothermic reaction. They are not contradictory.
Nitrous oxide and hibernation
I have read somewhere of an experiment where they submitted rats to a nitrous oxide (If I remember well) rich atmosphere and they entered a hibernation-like phase, for many hours, with decreased thermal regulation, heart beat etc besides consuming very little oxygen. They recovered, with no apparent damage to their brain or body, a few hours after the gas was switched off. It also said that the effect was apparently due to the temporary deactivation of the mitochondria, perhaps pointing to a inherent protective mechanism. I can't remember the link or find it again. Does anyone know about this research? DSedrez 00:13, 11 March 2006 (UTC)
This refers to work from a group in Seattle reporting a hibernating-like state induced by exposing mice to hydrogen sulfide (H2S) and not to N2O. Please see E. Blackstone, M. Morrison, M.B. Roth, Science 2005; 308: 518. ISMG, 22.34, 21 October 2007 —Preceding unsigned comment added by Ismg (talk • contribs) 20:35, 21 October 2007 (UTC)
Inhalation methods
Inhalation directly from a whipped-cream charger or a tank poses serious health risks, as it can cause the lungs to collapse from high levels of pressure, forcing air into the chest cavity, and can cause frostbite since the gas is very cold when released.
While this is true for direct inhalation off of a tank, inhalation from a whipped cream charger is quite different, as the bottle serves the same purpose as a balloon, mediating the pressure and temperature issues. I'm deleting the charger reference. El Mariachi 10:23, 5 June 2006 (UTC) Be careful about the usage of the term "charger". The charger refers to the metal container the compressed nitrous oxide is stored in, not the dispenser. The charger dispenses gas at high pressure and low temperature into a dispenser, from which it can be more safely consumed. Do not consume nitrous directly from a charger. 24.126.68.166 05:07, 8 July 2006 (UTC)
Legality contradiction
Legality section first states not illegal to posses - then in second paragraph says it is. can someone confirm which is correct?202.36.134.22 00:33, 6 June 2006 (UTC)
- The answer is highly dependent on jurisdiction, manner of packaging / sale, presence of adulterants, and intended use. I think the article does an acceptable job of explaining this. Do you have specific comments on the section or areas of confusion? Evand 16:27, 26 August 2006 (UTC)
Dipole moment
I'll put this here until the infobox is updated. Dipole moment of N2O: μ = 0.166 D
Ben 23:32, 7 August 2006 (UTC).
Monopropellant Isp
I get that monopropellant Isp is only 180s, not 200s (100:1 expansion ratio nozzle, frozen equilibrium -- a fairly standard metric for a vacuum nozzle). Do you have a reference? Also, there's a bit of a difference between 180s and the 235s of a hydrazine thruster. And "Just add gaseous hydrogen" is true but fairly irrelevant -- gaseous hydrogen tankage is really heavy, so there is no case where that would improve things from a system performance standpoint. Evand 16:33, 26 August 2006 (UTC)
Furthermore, pre-mixed nitrous oxide/hydrogen forms a dangerously unstable mixture even at very low concentrations of hydrogen. Maybe not a good idea to be suggesting this publicly? jonny.dyer
New subpage for recreational use
I have begun drafting a subpage for recreational use of nitrous oxide, because I think the current page doesn't satisfy recreational users or chemists. The draft can be seen and edited at User:Lamontacranston/recreational. Currently it is a condensed version of the current article, but I imagine if the current article gets a "See also" listing "Recreational use of nitrous oxide" the main article will then be able to cut out a great deal of content that is only interesting or useful to recreational users. I think that this will make the page more accessible for most users as well as help users who are coming to this page just to learn about recreational use. Please feel free to edit the draft and comment on my proposal. Lamont A Cranston 23:48, 19 September 2006 (UTC)
- Good idea. There's a precedent, Non-medical use of dextromethorphan, which you could use as a model. —Keenan Pepper 02:33, 21 September 2006 (UTC)
- Another precedent would be Effects of alcohol on the body. We could easily have an Effects of nitrous oxide on the body page, combining the medical and recreational uses of the substance; after all, they are closely related. We could even aspire to having the car/motor sport stuff on a separate page too, just leaving the chemistry of N2O (which is interesting enough) on this main article. Just a thought. --Guinnog 03:11, 21 September 2006 (UTC)
- I agree that the motorsports stuff should be on a subpage. I haven't made up my mind wether there should be separate medical and recreational pages. On one hand, they would overlap quite a bit (i.e., health concerns). On the other hand, where should "nitrous oxide use in popular culture" go? I'm leaning towards combining the two. Note for comparison that cannabis is a botany article; cannabis (drug) concerns recreational use, but has a section on medical use and a link to the article medical cannabis; and hemp describes non-drug cultivation and use. Lamont A Cranston 11:54, 21 September 2006 (UTC)
New infobox
I'm adding a new chembox. I'll put the old one here for archival, in case any info is needed from it.
Ben 21:28, 16 October 2006 (UTC)
|
General |
|
|---|---|
| Name | Dinitrogen oxide |
| Chemical formula | N2O |
| Appearance | Colorless gas |
|
Physical |
|
| Formula weight | 44.0 u |
| Melting point | 182.29 K (−90.86 °C) |
| Boiling point | 184.67 K (−88.48 °C) |
| Critical temperature | 309.6 K (36.4 °C) |
| Critical pressure | 7.245 MPa |
| Density | 1.2 g/cm³ (liquid) |
| Solubility | 0.112 g in 100g water |
|
Thermochemistry |
|
| ΔfH0gas | 82.05 kJ/mol |
| ΔfH0liquid | ? kJ/mol |
| ΔfH0solid | ? kJ/mol |
| S0gas, 100 kPa | 219.96 J/(mol·K) |
| S0liquid, 100 kPa | ? J/(mol·K) |
| S0solid | ? J/(mol·K) |
|
Safety |
|
| Inhalation | See main text. May cause asphyxiation without warning. |
| Skin | Hazardous when cryogenic or compressed. |
| Eyes | Hazardous when cryogenic or compressed. |
| More info | Hazardous Chemical Database |
|
SI units were used where possible. Unless otherwise stated, standard conditions were used. | |
- This article is about nitrous oxide, also known as NOS or laughing gas. For other meanings of laughing gas, see the disambiguation page.
Merging back in Effects of nitrous oxide on the body
I'd like to suggest that Effects of nitrous oxide on the body be merged back into this article. While keeping all the info on N2O's use as a drug maybe wasn't that bad an idea in theory, it's pretty much a mess with medical and recreational uses discussed on both this page and that page, some having information that the other doesn't but should, and it's attracted a lot of cruft such as the humongous "popular culture" trivia section (do we really need to know every single time a fictional character uses nitrous?) and a "sexual fetish" section that reads like an advertisement for a specific yahoo group. I'd like to suggest that all useful info in that article be culled back into this one. Krimpet 03:06, 25 February 2007 (UTC)
- Since nobody has objected, I have completed the merge. Krimpet 05:40, 17 March 2007 (UTC)
External link misdirects
According to Erowid.org, the Nitrous/Olney's Lesions link is not confirmed.
"Olney's Lesions: there is little to no evidence that Nitrous Oxide use causes the brain lesions described in William White's "This is your Brain on Dissociatives" and, without further evidence, this hypothesis should be considered invalid for Nitrous Oxide."
External link Nitrous Oxide chargers links to porn site www.amateurcurves.com.
Hazards
Someone should pore over the OSHA info for nitrous oxide available at http://www.osha.gov/SLTC/healthguidelines/nitrousoxide/recognition.html and inject it into the current article. It reveals various hazards of the chemical. 72.45.61.218 01:50, 1 May 2007 (UTC)
Chemical/physical
The reference provided by Wolfkeeper on 29-Jul-07 does not appear relevant to the question. The reference does not describe any accidents involving explosion of N2O, nor any issues regarding contamination of fuels. Charles 01:36, 30 July 2007 (UTC)
- as discussed in talk, the issue was dieseling of vaseline (petroleum jelly) on a hybrid rocket, and this issue is indeed pertinent. Basically, when nitrous oxide at high pressure is released into a pipe containing air and small traces of hydrocarbons, the air adiabatically compresses to high temperatures and ignites the hydrocarbons. This will then trigger the nitrous oxide to exothermically decompose with disastrous results (explosion).WolfKeeper 04:45, 30 July 2007 (UTC)
- So the explosion is a result of air and vaseline, not the N2O ? In that case the WP page for N2O needs to be amended to remove the references to N2O explosions, agreed?Charles 13:27, 30 July 2007 (UTC)
- No, the explosion is the result of nitrous self-decomposing after being ignited by dieseling. And as I stated, this was demonstrated in a hybrid test, it's not merely a theoretical problem. In fact, similar problems are common to many oxidisers.WolfKeeper 20:18, 30 July 2007 (UTC)
MSD?
Apparently someone is changing the infobox? I'm sure you've noticed that the MSD pointer is invalid for some reason. Was about to edit it when I discovered that there is some "trick" to the template with which I am not familiar. Apparently points automagically, in this case to an edit box in Wikipedia. Student7 23:54, 10 August 2007 (UTC)
"other propellants used in cooking spray include food-grade alcohol and propane"
I don't think "other propellants used in cooking spray include food-grade alcohol and propane" is correct. Alcohol is used as a solvent, not a propellant. Right? NCdave 08:37, 13 September 2007 (UTC)
yeah, ethanol (and every other alcohol) is liquid at room temperature and atmospheric pressure, it's not going to propel anything, 118.90.131.178 (talk) 13:37, 1 May 2009 (UTC)
Dipole direction in infobox
Is the image that shows nitrous oxide to be more electronegative near the noncenter nitrogen atom correct? Wouldn't the oxygen atom be more electronegative?
--76.27.237.214 05:07, 24 October 2007 (UTC)
Quality of Nitrous Oxide article
Doesn't Nitrous Oxide have the chemical formula of NO? N2O as used in the article is surely di-Nitrogen Oxide! i.e N-O-N. Apologies if I've made a silly mistake.
—Preceding unsigned comment added by 77.101.115.153 (talk) 18:06, 16 June 2009 (UTC)
Under "History" I find the word "[penis]". How did that get there?
This article is very informative, but still needs some cleanup. For example, dual spellings of anesthesia/anaesthesia (I vote for the simpler American/Canadian one vs. the British spelling). And occasional casual/informal writing style, inappropriate for this type of subject. —Preceding unsigned comment added by 64.223.205.36 (talk) 16:15, 27 November 2007 (UTC)
(speculation) Possibly that word was placed there in an attempt to trigger an automated censor into hiding the
article from someone's children. 118.90.131.178 (talk) 13:42, 1 May 2009 (UTC)
Recreational use
It states that "Since the earliest uses of nitrous oxide for medical or dental purposes, it has also been used recreationally as an inhalant, because it causes euphoria and slight hallucinations" I maybe mistaken but it was my understanding that NO2 was used recreationally long before it was used for medical or dental purposes. I'll try and find refs for this but if anyone knows more, please post. —Preceding unsigned comment added by 99.240.244.237 (talk) 02:24, 17 December 2007 (UTC)
Yes, you are correct. Humphry Davy introduced it recreationally around 1800, long before it was used medically. He called it "laughing gas" because at parties (attended by intellectuals, not lowlife-druggie types), everyone had a laughing-good time watching the fucked-up guy stumble around. It does NOT make you laugh or giggle. This is an important fact but I'm not putting it in because no one would believe it without a citation, but it's true. --TechnoFaye Kane 06:36, 19 November 2009 (UTC)
Mistake in Recreational use
It says "By allowing the gas to expand in a balloon, bag or a whipped cream canister, the final output temprature of the gas is lowered immensly."
The final temperature is immensly _increased_, compared to inhaling it straight from the tank, that's what the balloon is for —Preceding unsigned comment added by 153.19.42.120 (talk) 17:04, 15 January 2008 (UTC)
- Yeah, that sentence - while technically correct - is severely misleading, at best.
- When the gas is released from the cartridge, its expansion causes the temperature to dramatically drop. However, as soon as it is in the balloon or the canister, it quickly warms back up to room temperature. Like, practically immediately. And that sentence implies that somehow one has the option of not allowing the gas to expand, which is patently false. Firejuggler86 (talk) 06:05, 6 April 2021 (UTC)
Needless fud headers
Whoever claimed that the details of recreation use were unencyclopedic: lay off. This information is relevant to anyone studying drug abuse among young Americans, among whom use of the drug is rampant. Understanding the dangers associated with different methods of administration is also important to anyone using the drug. I find it hard to imagine that more people care about NO2's industrial synthesis than how thousands (millions?) of people routinely use it. Acone (talk) 18:32, 15 November 2009 (UTC)
agreed this information is probably the first information many readers are looking for. but while we're on the topic, this entire segment: Recreational abuse of the pure gas is also associated with negative health effects. A long term use, can lead to drug addiction. If the gas is inhaled for long-term use in excessive quantities, it has been associated with vitamin B12 deficiency anemia (due to reduced hemopoiesis), neuropathy, tinnitus, and numbness in extremities, unless vitamin B12 supplements are taken to counteract this. Chronic use among pregnant women has been shown to be teratogenic and foetotoxic. Neurotoxicity may also occur from the long term use of nitrous oxide. is worthless speculation (i.e. indistinguishable from do-gooder ranting to scare the kids) unless someone cares to supply some citations. N2O, like most drugs, is accompanied by urban legend about "known" harmful effects. in other words, without citation this material is better removed. note, especially, that "associated with" are weasel words that could mean anything, much like the Bush administration's enthusiasm for the expression "linked to" —Preceding unsigned comment added by Snaxalotl (talk • contribs) 12:58, 22 May 2010 (UTC)
Agreed. There's also an unnecessary comma. I'm removing the section. Cheezmeister (talk) 02:59, 1 June 2010 (UTC)
We need a more recent reference to be added
Used some time today to clean the chapter from unsourced info. Instead the main part of the chapter, is now based upon info from the reference "Consumers union report on licit and illicit drugs (1972)": http://www.druglibrary.org/schaffer/Library/studies/cu/CU43.html Havent searched for additional references to cover the chapter "recreational use". As the used reference is nearly 40 years old, it would be a significant improvent if some of you can find a new more updated reference, to describe the extend of the "recreational use" in 2000-2010. Havent got the time to look further into this. If some of you have knowledge of a new more updated source, then please go ahead and update the chapter. Just remember, that no info can be written without a proper reference being cited. :-) Danish Expert (talk) 14:23, 23 April 2010 (UTC)
Can a better term than "Recreational Use" be found?
The meaning in this article is more like "non-prescription drug use". Recreation would include off road vehicles or racing or spraying whipped cream on someone before... Well, the point is, recreation takes many forms and it is the use as a neuro-active agent that is being described. —Preceding unsigned comment added by 199.165.246.6 (talk) 19:43, 28 June 2010 (UTC)
Someone put some irrevelant and just plain wrong info in my mfg section
A gas is present in trace amounts in Earth's atmosphere as a result of high temperature reactions between nitrogen and oxygen. (THIS IS A)WRONG AND B) IRREVELANT TO MFG
The preparation is dangerous because of N2O's tendency to explosively decompose into nitrogen and oxygen at high temperatures. THIS IS LIKELY IS TOTALLY WRONG. WHEN AMMONIUM NITRATE EXPLODES, NOT MUCH N20 FORMS AND WHAT DOES WOULD NOT SIGNIVICANTLY EFFECT THE ENERGETICS
(The World Trade Center and Oklahoma City bombings involved detonation of nitrous oxide produced by rapid high temperature decomposition.) N2O manufactured this way should NOT be inhaled, because it is contaminated with NO2, a corrosive, irritating gas that can cause permanent lung and genetic damage. I INCLUDED PARAGRAPHS OF THE NOT TRIVIAL CLEAN UP REQUIRED FOR AMMONIUM NITRATE GENERATED N2O; SEE BELOW THIS DOES NOT BELONG HERE
SO THERE YOU HAVE THE DELETED ADDTIION AND WHY I GOT RID OF IT
PAUL S —Preceding unsigned comment added by 70.89.129.97 (talk) 22:33, 15 January 2008 (UTC)
Multiplied by 300 for what purpose?
This sentence -
"Human activity is thought to account for somewhat less than 2 teragrams (this is multiplied by about 300 when calculated as an equivalent amount of carbon dioxide) of nitrogen oxides per year, nature for over 15 teragrams."
- instructs one to multiply the mass by 300 to get the equivalent amount of CO2... but for what purpose? This needs to be deleted or explained.
Also, this sentence seems unnecessarily fierce:
"Nitrous oxide also attacks ozone in the stratosphere, aggravating the excess amount of UV light striking the earth's surface in recent decades,..."
This statement needs a credible reference to the connection between increasing amounts of atmospheric N2O and "excess" (whatever that means) amounts of UV light and ozone -- which has actually not changed much over the last decade[3].
-- Gouveia2 (talk) 21:53, 19 March 2008 (UTC)
Badly worded sentence
"Liquid nitrous oxide acts as a good solvent for many organic compounds; liquid mixtures and may form shock sensitive explosives." is badly worded, but I don't know what it's attempting to say. Needs fixing. -- Dougher (talk) 02:32, 29 May 2008 (UTC)
Nitrous Use for Women in Labor
I think this article could benefit from a section on the medical use for women in labor. N20 is used fairly commonly in several other Western countries as a mild alternative to other forms of pain relief, like epidurals.
- I agree.In Europe it´s one of the most commonly used pain reliefs for women in labour,easy to distribute and with few side-effects.A little less americanized view of the matter would be desireable.In Scandinavia I think more than 70% of all women use Nitrous oxide during child birth.This probably reduces the use of other "heavier" types of pain reliefs such as epidurals. —Preceding unsigned comment added by 84.216.32.190 (talk) 16:08, 11 December 2008 (UTC)
British vs. American English
As I was editing the two final sections of the article itself, I noticed that spellings and other usage mixed British and American English. The article seemed to begin or to be predominantly written in American English, so I changed everything (except for anaesthesia) to American English.
But then I got to thinking. Since Brits were the first to discover and experiment with nitrous oxide, shouldn't the entire article be written in British English?— Scrawlspacer (talk) 19:50, 21 July 2008 (UTC)
- Interesting angle though I wouldn't say that that constitutes strong national ties. Which was first? JIMp talk·cont 02:39, 4 July 2009 (UTC)
Climate change denial?
The "Occurrence" section seems to a bit of a Climate-change "denial" flavour to it. It needs more NPOV wording. In particular, just because water accounts for 95% of the earth's greenhouse effect it is not the case that therefore: "regulation has little, if any effect". Fig (talk) 22:20, 10 August 2008 (UTC)
- I think this has been removed. The current version reads correct and has no "denial" flavour to it to me. Hulten (talk) 15:32, 17 April 2020 (UTC)
N2O Denaturants
So the article states that automotive N2o is denatured with a sulphur compound to make inhalation unpleasant and/or impossible. I really think this is an old wives tale. There's no legal requirement to do this, so why would companies spend the money and open themselves up to liability? Here's an MSDS for automotive nitrous - it mentions nothing aside from N2O as the gases in the mix. It states >99% purity, which leaves 1%. If something had been intentionally added, that would need to be listed on the MSDS. https://pgw100.portal.gases.boc.com/boc_sp/nz/safety/0107.pdf
I propose that the statement about added sulphurus dioxide be removed, unless there are objections?
74.77.128.175 (talk) 02:52, 3 October 2008 (UTC)
I guess it got removed. However I have read elsewhere that there are other gases present in automotive nitrous oxide, not to discourage use in humans, but because it's not meant for humans (and the gases remaining from manufacture or existing for other reasons are not harmful to cars) or to add things that are also good for cars. It is therefore supposed to be highly dangerous to inhale automotive nitrous as opposed to food-grade or medical-grade nitrous which are purer. If this is incorrect then of course it shouldn't be in the article, but if it is correct it's important we put it back. I think we should find some good sources to resolve this issue. Rifter0x0000 (talk) 03:51, 31 January 2010 (UTC)
Energy Drink Use
I recently read an article and saw in the grocery store an energy drink containing nitrous oxide. does anyone know about this and what the effect would be of drinking it? Wolfyeb (talk) 13:30, 3 September 2009 (UTC)
This should be removed. As Nitrous oxide is a gas, you read wrong and the energy drink contained the ingredient -(NO)Nitric Oxide- recently discovered in 1999. As from the powder Arginine its precursor from. {No need to cite because it doesn't belong here} — Preceding unsigned comment added by 98.169.8.118 (talk) 20:46, 11 May 2013 (UTC)
Incorrect Bond Lengths in Picture
The bond lengths in the "ball-stick" picture at the beginning of the article appear to be incorrect. They imply that the average bond length between the two Nitrogen atoms is longer than the average bond length between the Nitrogen and Oxygen atoms. This is not the case as the Nitrogen to Nitrogen bond experiences resonance between a double and triple bond, whereas the Nitrogen and Oxygen bond experiences resonance between a single and double bond. I believe the Nitrogen, triple bond, Nitrogen, single bond, Oxygen compound is more stable, with respect to resonance structures, as Oxygen is more electrophilic. —Preceding unsigned comment added by 129.137.244.47 (talk) 15:02, 6 October 2009 (UTC)
- Thanks for pointing this out, I must have accidentally labelled the image the wrong way round when I made it.
- Fixed now.
Revert in section '"Laughing gas" (name)'
I've reverted an edit by User:TechnoFaye since it changed the article from reflecting the contents of the referenced homepage [4] into contradicting that homepage. It might be that User:TechnoFaye is correct about why Humphrey David named it "laughing gas", but in that case a reference needs to be added. That nitrous oxide never makes the user laugh is not true. Aenar (talk) 23:39, 29 November 2009 (UTC)
California has banned the sale of nitrous oxide to minors.
I think this should go somewhere in the legality section, but I'm not sure how to edit it in. Source is here: http://www.leginfo.ca.gov/cgi-bin/waisgate?WAISdocID=2791132412+0+0+0&WAISaction=retrieve Papre (talk) 07:10, 12 January 2010 (UTC)
Merger of Whipped-cream charger and Nitrous oxide ?
Someone (not me) has added a proposed merge tag on Whipped-cream charger (saying it should be merged with Nitrous oxide), but didn't add anything about it on the talk pages. The discussion link goes to the nitrous oxide article, but there's nothing there. I don't think the article needs to be merged, and I wonder if the person adding the tag is going to claim consensus without having actually instigated a discussion. Either way, something should have been added to the talk pages for these articles, so I am doing it now. Discuss. :D Rifter0x0000 (talk) 03:33, 31 January 2010 (UTC)
Someone (also not me) slapped this article with a WP:NOR tag and did not bother to put anything on the talk page. I'm not sure what's up with these drive-by tags. We should adhere to WP:NOR but it would have been nice if whoever tagged the article at least put something on the talk page about doing it and better yet said what they considered to be Original Research. I'm not saying that OR is not present in the article, I haven't looked at it that closely. But if there is some we should discuss replacing it with properly sourced statements or removing it, rather than just throwing tags around. Rifter0x0000 (talk) 04:28, 31 January 2010 (UTC)
"Whippits?"
There's a picture of a hand holding five canisters, labeled "whippits", but no mention of whippits in the article. —Preceding unsigned comment added by 65.10.29.182 (talk) 03:24, 20 March 2010 (UTC)
- Removed unexplained image. Materialscientist (talk) 09:52, 20 March 2010 (UTC)
Laughing effect
Could someone add a section on how N2O makes you laugh? I know that it somehow numbs the Reticular_formation, but I'm not exactly sure. Most people come to this article in the hope of finding how N2O makes you laugh. Thanks, ManishEarthTalk • Stalk 15:10, 21 March 2010 (UTC)
History
This week I wrote and added a new history chapter for the wiki article, with 9 new references. Just want to add here at the discussion page, that several of the references were found to be inaccurate when noting the exact year/time for certain developments. The years reported in the wiki article have therefore all been double checked, to get rid of those mistakes. Just to mention an example, its a written fact that Priestly discovered oxygen in 1771 and nitrous oxide in 1772, and then reported these findings in his book from 1775, that he revised in a more compact version in 1776. Another example is, that Wells in his own manifest claim he discovered the method of using the gas for anesthesia in Nov.1842, but all other sources agree to cite Dec.11-1844 as the first time the gas was used for anesthesia in a dental extraction, and therefore this exact month has been reported in the wiki article.
Status for the history chapter:
The history chapter has now fully described the: "Early use (incl. recreational use)" and "Anesthetic use".
If some of you have other relevent historic info to add, relating to the use of nitrous oxide for aerosol cans or supplement in rocket/automotive fuels, I suggest we place this info in a new History subchapter named "other use". In my point of view, the criteria to add any such additional info in a seperate history chapter, should however be, that the history had significant value for the field where nitrous oxide was used (or lead to a significant usage of nitrous oxide; to impact the worlds overall demand). If this is not the case, I vote that no such supplementary history chapter should be added. In example, I believe the use of nitrous oxide as aerosol in whipcream cans doesnt qualify to get mentioned in a seperate history chapter. Since I believe the use of nitrous oxide used for this purpose is very small, and also consider it didnt have a significant impact towards the total sale of whipcream, its not really worthy to highlight in a seperate history chapter. A short note of its introduction in whipcream cans, is in that case more appropriate just to briefly mention in the "applications" chapter instead.
Danish Expert (talk) 15:26, 23 April 2010 (UTC)
Errata
1.) re toxicity, anesthesia at childbirth. Study had mice gestating for few months in N2O/O2 90%:10%, results were low birth weight (low O2) but no negative physical changes to mice or offspring. 2.) Full (vision turns into a tunnel like looking into a cardboard tube) anesthesia (or abuse of N2O) achieved at 95% N2O. And things do seem funny after. The 30% mixture will certainly take the edge off pain and anxiety especially for a dental procedure (where it appears more effective for dental pain than opiates). Need 90% plus for amputations etc and air is 21% Oxygen so therapeutic safety range was non-existent in the old days, patients died of asphyxiation. Hence the nitrous-ether thing. 3.) Humans will get a "choking feeling" inhaling pure Argon or pure Nitrogen (inability to get oxygen) however that does not occur with Nitrous Oxide. 4.) #2 and #3: Was common for misleadingly high rate of suicides by dentists, putting mask over face with air or no O2. 5.) see wiki on Xenon and Xenon anesthesia, confirms higher Nitrous Oxide concentration for full anesthesia. Xenon is chemically unreactive in the body, yet elicits the same anesthetic action. This is due to the solubility in lipid membranes, notably the myelin sheath, and modification of membrane properties. See http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1J-3XNK10T-T&_user=10&_coverDate=09%2F17%2F1999&_alid=1327673582&_rdoc=5&_fmt=high&_orig=search&_cdi=4892&_sort=r&_st=5&_docanchor=&_ct=2807&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=903927f49c0fa186c7b1e38c6ec3845b 6.) note MSDS that Nitrous Oxide has a "sweet taste" which is one reason for preference as propellant for Whipped Cream, other is its high solubility in butter fat. Air from the bubbles in homemade whipped cream does not dissolve in fat well and hence does not deflate as quickly. the main danger of inhaling nitrous oxide from whipped cream containers is the high risk of pneumonia from mist of fat globules being inhaled into the lungs 7.) Commercial grades of nitrous oxide, like for welding and street racing, contain nitric oxide(NO/N2O2) and nitrogen dioxide(NO2/N2O4) (nitric oxide outside the body reacts rapidly with oxygen, including in your lungs, to form NO2), the NO2 forming Nitric Acid with moisture and other reactions with organic compounds (DNA mod by nitrosylation/deamination, nitration of unsaturated lipids, et al). Exposure causes pneumonia. 8.) Potassium Nitrate and Ammonium Sulfamate (a weed killer) worked nicely, like with all synthesis of N2O we filtered through wet iron wool (not steel wool) which removes Nitric Oxide. The products of all synths do contain a trace of Nitrogen. 9.) As a reducing agent wouldn't Sulfur Dioxide be a detonation hazard if added to Nitrous Oxide, as well as Sulfuric Acid be bad for your engine? 10.) In racing carburetion you get 1 volume of oxygen for 2 volumes of Nitrous Oxide or 4 volumes of air. Like a turbocharger. 11.) In amateur rocketry a simple and easy to handle fuel is better than power. For aircraft like Me-163, Aqueous Hydrogen Peroxide and Aqueous Methanol generated low-temp (low velocity) high volume (more momentum) exhaust good for 600 MPH plane going on a short hop. The limiting factor for a real rocket is weight of Oxidizer not weight of Fuel. The Rocket Research Hydrazine (instant reaction on contact with Iridium Catalyst)thrusters for NASA vehicles. The reactors are easy to conrtol; fuel can't detonate. Energy from creating N2 triple bond (~250 Kcal/mole) and 2 H2 (104 Kcal/mole). Energy from decomposition of N2O is less than 150Kcal/mole on par with Hydrogen Peroxide(which like N2O and Nitromethane, detonates). Approximating since high Temps (N2O decomposes ~550 C but reaction at 1500 C) alter reaction. Carbon burns less efficiently above 1200 (equilibria C + CO2 = 2 CO)(lower temp 800C candle flame: C > CO 85% energy; CO > CO2 15% energy) and water begins to separate above 1800C (from Solar hydrogen generator info)(big NASA H2 O2 engines power from Hydroxyl generation). 12.) Nitrous Oxide is generated by the human body as a side product of Nitric Oxide Synthase enzyme. 13.) N2O is generated by de-nitrification of soils (a consequence of man's use of high nitrogen fertilizer) http://www.dndc.sr.unh.edu/papers/JGR1992a.pdf 14.) N2O has similar critical constants to CO2, not sure how adiabatic compression generates that much heat. 15.) I've used whippets in by BB guns, in place of CO2 cartridges. 16.) Hottest flame, used in my profs Atomic Absorption Spectrometer is Nitrous Oxide and Cyanogen at 2500 degrees C.
http://www.airproducts.com/nr/rdonlyres/8c46596e-2f7d-4895-b12a-e54cd63e1996/0/safetygram_20.pdf "Published data confirms that animals exposed to atmospheres containing ≤ 500 ppm nitrous oxide show no evidence of any reproductive effects."
MSDS: https://apdirect.airproducts.com/msds/DisplayPDF.aspx?docid=70704 Thermal decomposition at 575°C.
Nitrous oxide decomposes exothermically (ΔHR = -81.6 kJ/mol N2O).... heat input is required to initiate the reaction. In the case of thermal decomposition, the activation energy barrier for nitrous oxide is about 250 kJ/mole. Rocket engine, catalysis:
http://pdf.aiaa.org/preview/CDReadyMJPC2005_1177/PV2005_3919.pdf
Shjacks45 (talk) 21:00, 9 May 2010 (UTC)
- I don't know about inhaling pure argon, but it is my understanding that inhaling pure nitrogen does not cause a person to experience a choking feeling. Inhaling a mixture of 70% carbon dioxide 21% oxygen and 9% nitrogen will cause a person to feel they're choking, even though they are receiving sufficient oxygen, due to the presence of carbon dioxide. Firejuggler86 (talk) 06:43, 6 April 2021 (UTC)
Used as an inert gas for packaging
Isn't the statement, that N2O is used as an inert gas for packaging potato chips false? As far as I know, nitrogen, not N2O is used for this purpose. The potato chip page talks about nitrogen gas as well: "Today, chips are packaged in plastic bags, with nitrogen gas blown in prior to sealing to lengthen shelf life, and provide protection against crushing."
Does anyone has a citation for the N2O statement? --Sdfx (talk) 12:46, 11 August 2010 (UTC)
- Yes, I think you're correct and the statement about potato chip bags is false. Here's a source that indicates that nitrogen, not N2O, is used in that case: "In fact, bags of crisps are not filled with atmospheric air but nitrogen, because the oxygen in air would cause the crisps to go soft. Nitrogen gives the product a longer shelf life, and a 1994 study suggested that it makes the crisps tastier." (http://www.bbc.com/future/story/20151105-im-fed-up-with-all-the-air-in-my-crisps-and-this-is-why) I'll remove the incorrect statement from the article. PGibbons (talk) 21:10, 8 September 2018 (UTC)
File:Nitrous oxide - 10 x 8g.jpg Nominated for Deletion

|
An image used in this article, File:Nitrous oxide - 10 x 8g.jpg, has been nominated for deletion at Wikimedia Commons in the following category: Deletion requests August 2011
Don't panic; a discussion will now take place over on Commons about whether to remove the file. This gives you an opportunity to contest the deletion, although please review Commons guidelines before doing so.
This notification is provided by a Bot --CommonsNotificationBot (talk) 12:36, 9 August 2011 (UTC) |
Banning NO2 recommendation resource
http://www.bloomberg.com/news/2011-12-06/carbon-credits-becoming-junk-before-2013-ban-closes-door-energy-markets.html by Dinakar Sethuraman and Natalie Obiko Pearson Bloomberg.com Dec 6, 2011 1:02 PM ET
"HFC-23 (hydrofluorocarbon-23) and NO2 (nitrous oxide) are so damaging to the environment that they should be banned outright and not entitled to get emission credits, the EU and other governments have said. "
99.190.82.160 (talk) 07:47, 7 December 2011 (UTC)
Vitamin B12 Interference Source Proposal
Hi. I am not familiar with how to make citations in Wikipedia, but I think I found what would qualify as a source for the [citation needed] tag under the Vitamin B12 Interference subheading. Assuming it is valid, can someone add it please? http://jmt.pennpress.org/PennPress/journals/jmt/sampleArt3.pdf Redex777 (talk) 05:05, 8 April 2012 (UTC)
new trends in recreational nitrous use
I recently read about a product called "Das Whippet Meister" in a head shop. Its screwed onto a bottle of nitrous and filters the gas with a barb for balloons on the end. I feel that this product and the information i put in the same section regarding wet chemistry are very relevant to the article. Please do not delete this section as im sure a lot of people will find it very interesting and or useful. — Preceding unsigned comment added by Chrisgedwards (talk • contribs) 01:44, 25 April 2012 (UTC)
Suggestion: Remove the "empty bottle"-picture from the article infobox.
I suggest removing the picture of an empty Duran bottle (claiming to have N2O gas in it) from the N2O article. I cannot see that this picture contributes anything to the article, and I'd hate to see all Wikipedia articles about colorless gases and farts have this bottle added. The standard infobox-property "Appearance" says it's a "colorless gas. A picture of a bottle with or without something invisible in it seems redundant.
Tungstic (talk) 20:31, 30 May 2012 (UTC)
12-15 ?
This is a very odd sentence:
"Nitrous oxide has been used for anaesthesia in dentistry since December 1844, where Horace Wells made the first 12–15 dental operations with the gas in Hartford."
is that number really so significant that it must be included even though it seems not to be actually known? — Preceding unsigned comment added by 23.119.205.88 (talk) 03:18, 25 November 2013 (UTC)
Greenhouse / climate impact of nitrous oxide
I was looking for information on the climate-change impact of N2O. The page does provide some good info on that. Might someone be able to talk about how N2O is removed from the atmosphere over time? What is the 'half life' of the gas in the atmosphere? Barryazx (talk) 18:11, 1 July 2014 (UTC)
Pre-ignition... spelling in English
Is there really a difference between pre-ignition and preignition in rocketry ? Both spellings are in this article once.--Dartelaar [write me!] 12:53, 16 August 2014 (UTC)
- There is not a difference. Hyphenation is largely a question of style but a given article should be conistent. As the Wikipedia article has settled on pre-ignition (Engine_knocking#Pre-ignition) I will change the article to do that consistently.--NHSavage (talk) 18:55, 16 August 2014 (UTC)
Lacks
Lacks mention of adverse and dysphoric reaction in small percentage of population.
NO2 safety during pregnancy
I have a recommendation to add to the page. In the safety section of the content it may be a good idea to add information regarding NO2 and pregnancy. Pregnant women, especially in the 1st and 2nd trimesters, should not be in the room during the administration of NO2 due to the harm it can cause the fetus.
Lepeule, J., Caini,F., Galineau, J., Hulin, A., Marquis, N., Bohet, A., Thiébaugeorges, O., Goua, V., Kaminski, M.,Charles, M.A., Slama, R. (2009, November). Maternal Exposure to NO2 During Pregnancy and Fetal Growth: A Comparison of Two Exposure Models. Epidemiology 20(6)s79. Retrieved from http://journals.lww.com/epidem/Fulltext/2009/11001/Maternal_Exposure_to_NO2_During_Pregnancy_and.206.aspx (Allysonmarthaler (talk) 18:58, 30 July 2015 (UTC))Allysonmarthaler
- This seems rather specialized. It's not as if people are normally swarmed by N2O molecules are they? Btw the formula is N2O, not NO2. 2A02:8388:1600:6900:BE5F:F4FF:FECD:7CB2 (talk) 13:17, 23 September 2015 (UTC)
Contraindications
There are a few condraindications when using Nitrous oxide. Caution should be used with pregnancy also with patients who have a methylenetetrahydrofolate reductase(MTHFR) diagnosis. There have been links to fatal outcomes for individuals who use nitrous oxide sedation and have a MTFHR diagnosis. A study that was conducted showed results of those who have MTHFR are at an increased risk of developing abnormal plasma homocysteine concentrations post nitrous oxide sedation. To read this research study go to http://www.ncbi.nlm.nih.gov/pubmed/18580170. (Allysonmarthaler (talk) 20:27, 6 August 2015 (UTC))
Issue with "Nitrous can use stainless steel and aluminum as fuel."
From the info in citation 66, it appears that aluminum and stainless steel fuels are mentioned for rhetorical purposes, and nowhere in the article are they claimed as actual potential fuel sources for Nitrous oxide. Although adiabatic ignition is possible, this is not due to reactions with aluminum or stainless steel container walls. However, copper and iron tubing should be avoided due to their oxides catalyzing N2O's decomposition. — Preceding unsigned comment added by 130.18.104.130 (talk) 18:21, 26 August 2015 (UTC)
External links modified
Hello fellow Wikipedians,
I have just added archive links to 4 external links on Nitrous oxide. Please take a moment to review my edit. If necessary, add {{cbignore}} after the link to keep me from modifying it. Alternatively, you can add {{nobots|deny=InternetArchiveBot}} to keep me off the page altogether. I made the following changes:
- Added archive https://web.archive.org/20141219051822/http://journals.lww.com/anesthesiology/citation/1941/07000/Technical_Development_of_Gas_Anesthesia.4.aspx to http://journals.lww.com/anesthesiology/citation/1941/07000/Technical_Development_of_Gas_Anesthesia.4.aspx
- Added archive https://web.archive.org/20110124034306/https://unfccc.int/essential_background/convention/background/items/1362.php to http://unfccc.int/essential_background/convention/background/items/1362.php
- Added archive https://web.archive.org/20131127131246/http://sanghioverseas.com/nitrous_oxide_gas_plants/nitrous_oxide_gas_plants.htm to http://www.sanghioverseas.com/nitrous_oxide_gas_plants/nitrous_oxide_gas_plants.htm
- Added archive https://web.archive.org/20150108000814/http://journals.lww.com/anesthesiology/citation/1941/09000/The_Development_of_Anesthesia.8.aspx to http://journals.lww.com/anesthesiology/citation/1941/09000/The_Development_of_Anesthesia.8.aspx
When you have finished reviewing my changes, please set the checked parameter below to true to let others know.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers. —cyberbot IITalk to my owner:Online 01:04, 28 August 2015 (UTC)
Environmental
The current Environmental subsection mentions the USA but not other areas of the world. The world is larger than the USA. That subsection should instead mention other countries as well - or get rid of the USA-centric part. Additionally, the article should primarily focus on N2O in itself, and only secondarily on its environmental impact (the latter one could be gathered in the greenhouse-article for example) 2A02:8388:1600:6900:BE5F:F4FF:FECD:7CB2 (talk) 13:15, 23 September 2015 (UTC)
Assessment comment
The comment(s) below were originally left at Talk:Nitrous oxide/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| Comment(s) | Press [show] to view → |
|---|---|
The following suggestions were generated by a semi-automatic javascript program, and might not be applicable for the article in question.
| |
Last edited at 17:00, 3 February 2007 (UTC). Substituted at 01:20, 30 April 2016 (UTC)
As a byproduct
In that section, it says," The synthesis of adipic acid; one of the two reactants used in nylon manufacture, produces nitrogen oxides including nitric oxides[85][86] This might become a major commercial source, but will require the removal of higher oxides of nitrogen and organic impurities. Currently much of the gas is decomposed before release for environmental protection."
In calcium nitrite, "Calcium nitrite can be produced by different synthesis processes. One is by reacting hydrated lime with NOX gas, which typically comes from a nitric acid plant... It has to be kept in heat-proof place, because when the temperature is higher than 220 °C it will reduce and decompose into nitrous oxide." If this could still be done, then using hydrated lime could absorb the higher oxides, leaving nitrous oxide. Then the nitrite is decomposed to nitrous oxide. Could the enemy come from oxidizing the organic impurities to carbon dioxide ? -- Mountainninja (talk) 03:54, 20 June 2016 (UTC)
Alleged "contradictions"
I removed the "contradictions" template and the following paragraph from the lead:
- Nitrous oxide gives rise to nitric oxide (NO) on reaction with oxygen atoms, and this NO in turn reacts with ozone. As a result, it is the main naturally occurring regulator of stratospheric ozone. It is also a major greenhouse gas and air pollutant. Considered over a 100-year period, it is calculated to have between 265 and 310 times more impact per unit mass (global-warming potential) than carbon dioxide.[1][2]
Reasons: (1) Messages to other editors should be in the talk page, not in the article itself. If an editor has grounds to suspect that an information is incorrect, and she cannot find reliable references, she should move it to the talk page and let others provide such references, if they exist. (2) The links to the references given are broken. (3) The main claim in that paragraph is incorrect: while N
2O has greater greenhouse power than CO
2 per unit mass, its concentration in the atmosphere is very small, so that its contribution to the greenhouse effect is negligible -- as explained in greenhouse gas.
The first sentence seems to be correct, and the second one may be too; but they should go to the proper section in the article, with its effects properly compared to man-made ozone scavengers. --Jorge Stolfi (talk) 18:13, 14 February 2017 (UTC)
- Restored the first two sentences with clearer wording and a working reference. --Jorge Stolfi (talk) 18:50, 14 February 2017 (UTC)
Environmental section cleanup
Removed this paragraph from the "Environmental" section, since it is not specifcally relevant to the article. It may be appropriate for some article on environmental policy or grenhouse gases.
- The United States of America signed and ratified the United Nations Framework Convention on Climate Change (UNFCCC) in 1992, agreeing to inventory and assess the various sources of greenhouse gases that contribute to climate change.[3] The agreement requires parties to "develop, periodically update, publish and make available... national inventories of anthropogenic emissions by sources and removals by sinks of all greenhouse gases not controlled by the Montreal Protocol, using comparable methodologies...".[4] In response to this agreement, the U.S. is obligated to inventory anthropogenic emissions by sources and sinks, of which agriculture is a key contributor. In 2008, agriculture contributed 6.1% of the total U.S. greenhouse gas emissions.<ref name="epa.gov"/>
I also removed this sentence since it is not clear whether the increase refers to total agricultural emissions of greenhouse gases (chiefly CO
2 and CH
4) or to N
2O specifically:
- Additionally, estimated emissions from agricultural soils were 6% higher in 2008 than 1990.[3]
--Jorge Stolfi (talk) 18:34, 14 February 2017 (UTC)
Unclear sentence about N2O/O2 gas mixing
I could not understand this sentence:
- The medical grade gas tanks contain a mixture with 50%, but this will normally be diluted to a lower percentage upon the operational delivery to the patient.
Percentage of what? Diluted with what? If appropriate, please clarify and return to the "Medical use" section. --Jorge Stolfi (talk) 02:37, 15 February 2017 (UTC)
- ^ "Overview of Greenhouse Gases – Nitrous Oxide" (PDF). US EPA. Page 164 (document header listing). Retrieved 19 March 2014.
- ^ "U.S. Greenhouse Gas Inventory Report: 1990-2013" (PDF). US EPA. April 2015. page 60. Retrieved 19 August 2015.
- ^ a b "2011 U.S. Greenhouse Gas Inventory Report | Climate Change – Greenhouse Gas Emissions | U.S. EPA". Epa.gov. Retrieved 11 April 2011.
- ^ "FULL TEXT OF THE CONVENTION, ARTICLE 4(1) (a)". Unfccc.int. 31 December 1998. Archived from the original on 24 January 2011. Retrieved 11 April 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)