Talk:Adenosine triphosphate/Archive 1
| This is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 |
PLEASE
- Actually, there is a place to ask questions like this, just check out the Reference desk. --Lexor|Talk 14:07, Nov 9, 2004 (UTC)
- I stand corrected -- I wasn't aware of the reference desk. However, I hope you'll agree that the original poster's question doesn't go here. Zack
- Yes, it shouldn't go here, I always direct such questions to the reference desk. --Lexor|Talk 13:07, Dec 30, 2004 (UTC)
- I would argue that even the reference desk is not a place for homework questions. If the questioner has got this far it does not seem unreasonable to expect them to read the articles. At the reference desk it is appropriate to answer by pointing out the relevent article but not to give the answer. I'm not saying we should not give help in finding the answer but just handing it over seems a little lame. Some questions at the reference desk are obviously not homework so i would normally be happy asnwering such questions. Obviously it is a bit subjective trying to draw the line between the two. David D. (Talk) 19:09, 30 December 2005 (UTC)
- Yes, it shouldn't go here, I always direct such questions to the reference desk. --Lexor|Talk 13:07, Dec 30, 2004 (UTC)
- The way a librarian would do this to say, Please ask questions of this sort at the reference desk, but since you;re already here, I'll help you this once. The articles (and sections) you should read are .... Start with the..."
The key part of this is"just this once"
Formatting comment
Firefox's view of this articles is fugged'up. The top image and chart are streched till the middle of the left panel.
- Fixed, thank you. TimVickers 22:09, 11 April 2007 (UTC)
4 Oxygen version
I have heard that some plants have a 4 oxygen version of ATP. Can someone add a paragraph about this. I Know this is true cos I have a botanist friend completeing a PhD about eveolution of grasses in relation to 3-ATP and 4-ATP Robin48gx (talk) 11:57, 8 March 2013 (UTC)
GTP vs ATP
I dont understand why is GTP used in some reaction in place of ATP eg. in gluconeogenesis.
- While ATP and GTP are energetically equivalent (i.e., the same amount of energy can be harnessed from ATP as from GTP), their use in biochemical reactions is enzyme-dependent. Some enzymes, such as succinyl-CoA synthetase, use GTP to drive catalysis. Others, like hexokinase, require ATP instead. Which nucleotide gets used depends heavily on the specificity of the enzyme in question, which in turn depends on the enzyme's amino acid composition and secondary/tertiary structures.
- Also, for what it's worth, the production of GTP instead of ATP by certain enzymes is inconsequential since GTP can be converted to ATP via substrate-level phosphorylation. --Diberri 22:54, 2 Mar 2004 (UTC)
- I believe there is also an anabolic/catabolic and protein/fat/carbohydrate/nucleic acid distinction at work in there as well. My guess is there is separation to allow the cell to target energy resourses to specific pathways. Can anybody confirm this? --Anonymous
- Okay, I got an answer: there is no functional distinction between ATP and GTP. As per Dr. Robert S. Haltiwanger, Department of Biochemistry and Cell Biology, State University of New York at Stony Brook:
- There is no logical reason why ATP is used over GTP. It's like asking why do we only use L-amino acids instead of D-amino acids. You can ask God when you get to heaven.
- There are several reactions that use GTP in preference over ATP, such as succinyl-CoA synthetase, but there does not appear to be any clear distinction as there is for NADH and NADPH.
- This is partially untrue. While the reason for ATP's role difference over GTP is unclear (in terms of etiology; and may be arbitrary) there *is* a clear distinction between the usage-modes of ATP vs. GTP. Outside of reactions where they are used as synthetic accessories, ATP is used primarily as an energy exchange medium; the breaking of ATP's phosphodiester bonds are used to perform irreversible work, and the ADP is typically immediately released by the enzyme involved in the process so that it may be recycled. HOWEVER, the energy released by GTP is used to irreversibly change the conformation of the enzyme, sort of a molecular switch. The resultant GDP remains bound to the enzyme, altering its conformation, and thus its function. Later, accessory proteins called GEFs (GDP/GTP exchange factors) remove the GDP and allow the process to recycle. So in this case energy is used to ratchet the enzyme through three states - apo, holo-gtp, and holo-gdp. The conformations (and functions) are different in all three states, and the halflives of each state are carefully titrated by evolution to enable the molecule to correctly perform its function. Thus GTP is not merely an "energy store" but an accessory for molecular switching. UTP in eukaryotes has a general use as a transport handle; small molecules are often tagged with UDP to get them across certain lipid bilayers. "alternative" (i.e. non-rna-synthesis) roles for CTP have been less well explored.
Copyvio
I removed a section added by User:Jerryseinfeld it was a copyvio: a series of verbatim paragraphs from http://www.hussman.org/fitness/. --Lexor|Talk 13:07, Dec 30, 2004 (UTC)
I noticed that the site has a statement: "Brief quotations which include attribution and a link to this website are authorized for noncommercial use." Nevertheless, I removed it because:
- it was more than a brief quotation (several consecutive paragraphs)
- the GFDL does not restrict commercial use, and we don't encourage the addition of material that prevents commercial reuse or distribution.
So i'm wondering, since ATP is basically how cells store energy, would it be possible to just eat a bunch of ATP's directly and get some energy from them? :) SECProto 01:59, Feb 25, 2005 (UTC)
- It'd be more fun to eat a bunch of LSDs :-) --69.234.183.71 01:09, 19 Mar 2005 (UTC)
- I'm no biochemisty, but I expect that the ATP would undergo hydrolysis before it made it to the muscles. This article also states, "ATP cannot be stored, hence its consumption must closely follow its synthesis." so it looks like shooting back those little coffee-shop sachets of sugar is the best you can do. --Maelin 03:59, 28 December 2005 (UTC)
- ATP is far too unstable to just be eaten and consumed. It's far more efficient just to consume sugars and carbohydrates and derive energy from them. – ClockworkSoul 12:50, 28 December 2005 (UTC)
- ATP cant be stored as such so "eating it" would be pointless, furthermore it is constantly being utilised by the body so the effects would be very short term. Further to that the body would use its ATP in ~3s during very intense explosive exercise (ie first 10m of 100m sprint), but rapid regeneration occurs via creatinephospate (ie ATP+CP energy system). You can increasine CP stores in the muscle via creatine supplementation and in fact that is what weight lifters and body builders do, it is a proven ergogenic aid. hope that helps somewhat. StrengthCoach 20:41, 5 February 2006 (UTC)
Adenosine (Not ATP)
Reference to my last question, it is adenosine that causes ATP to lose its Phosphates and turn into normal adenosine (which is now thought to be a factor in determining sleep cycles)(I think) See Adenosine and Sleep for further reading
pKa
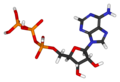
I think that the chemical structure featured in the image may be incorrect. All of the sources I can find show the terminal phosphate of ATP as being dehydrogenated and bearing a -2 charge at physiological pH. Does anybody happen to know the pK1 of ATP? – ClockworkSoul 21:21, 29 January 2006 (UTC)
form that often reacts with enzymes; bearing a -2 charge and complexed with something like Mg+2. Without a divalent cation, net 4 negative charges.
I'm not sure how the divalent cations inside cells influence this, but the pka for the second ionizing group on the terminal phosphate is about 7 and this source says: the overall charges of ATP and ADP at physiological pH are -3.5 and -2.5 --JWSchmidt 22:05, 29 January 2006 (UTC)
- Ah, good: I'm not crazy. In that case, given the best available information, perhaps we should remove that terminal hydrogen from the chemical structure image, and assign that terminal P the proper charge of -2, for a net of -4? – ClockworkSoul 05:57, 30 January 2006 (UTC)
- I just changed the picture in commons. see what you think. i can adjust it if needed. Or go ahead an make other modifications as needed. David D. (Talk) 06:14, 30 January 2006 (UTC)
- Looks great to me. I'm running out now so I don't have time to upload it to en, but I'll get it when I get home later if nobody else beats me to it. – ClockworkSoul 14:11, 30 January 2006 (UTC)
- The leaving of the first proton (any of the four in the "phosphate" part) gives a net (-) charge to the whole molecule wich makes it dificult for a second proton to leave, thus resulting in a higher (or lower, depending how you look at it) pK2 value. The same applies for the third leaving proton - its pK3 is even higher (lower). Without any cations only two of the phosphate -OH groups would dissociate, inside the cell the divalent cations (mostly Mg2+) would shield the charges resulting from the leaving of the first 2 protons bringing the net charge to 0 - this will allow the last two -OH groups to dissociate. - Boris 18:55, 10 February 2006 (UTC)
ER vs Wikipedia
I AM NOT AN EXPERT ON BIOCHEMISTRY, but I just watched the most recent episode of the TV show ER on TiVo and the numbers they quoted disagree with this article. On ER a "biochemistry professonr" said that there was 1/2 lb of ATP in a human body (I assume that means ADP + ATP since they are inter-converted) and he also said that each molecule was recycled 300 times per day. If I understand this article, the body has 0.1 mole x 507 g/mole = 50.7 g which is less than 1.8 ounces instead of 8 ounces. Also this article says ATP is recycled 2000 to 3000 times per day instead of 300. I doubt that the writers of ER just made up these numbers - I hope they came from some expert. So what do the experts on Wikipedia say about this discrepency? - FrankH 04:50, 7 February 2006 (UTC)
- These are the numbers I have at hand for an average size human at rest. I can drag out the reference if you are interested. This does not help with your figures above
- 9 x 1020 molecules of ATP are metabolised each second.
- 65kg of ATP produced each day per person (obviously we don't gain weight this is reflected in the high turnover of the molecule.
- Consume 700g glucose
- Excrete 1kg carbon dioxide
- Given those numbers 65,000/50.7 would suggest that the turnover (recycle) is 1300. Given my figures are for at rest the wiki article would appear to be in the right ball park. If the quantity of ATP is nearer 220g, as suggested by ER, then the turnover rate would be 300 as the show suggests. So the disputed figure here is the total ATP in the body (I would argue that is ATP, ADP and AMP). I suspect ER had the correct amount of ATP turned over each day but overestimated the amount of ATP in the body. David D. (Talk) 06:19, 7 February 2006 (UTC)
Also a suggestion for section "1 Chemical Properties". Figures of -12 kCal/mole and -7.3 kCal/mole mean nothing to me, so when it says "This massive release of energy...." it would be very helpful if there were a comparison that would be more meaningful to me. For example, compare the energy content of common sugar or fat per gram to the energy content of ATP per gram. Any expert able to supply this kind of comparison? - FrankH 04:50, 7 February 2006 (UTC)
- It is not that simple and it is a misrepresentation to say there is a massive release of energy. That needs to be changed. In fact ATP the terminal phosphoanhydride bond is an intermediate energy bond. Also you seem to be equating the energy of ATP with the whole molecule but we are only dealing with one chemical bond in ATP. The -7.3 kcal/mol (or -12 kcal/mol depending on the physiological concentrations) above is the free energy available for work after the hydrolysis of ATP to ADP. I could tell you that glucose has almost 686 kcal/mol for the oxidation of glucose to carbon dioxide and that provides enough energy to synthesis about 36 ATP molecules from ADP. Since the synthesis of ATP would require at least + 7.3 kcal/mol the energy required would be 36 x 7.3 = 263. Consequently not all the energy from glucose oxidation would be used and there is an efficiency of about ~40% (actually very good compared to most machines). I think this is partly what you were hoping for although i do not think it is what will make it more meaningful.
- The key is to compare the free energy available from the hydrolysis of ATP to ADP compared to other similar reactions. The hydrolysis of glucose 6-phosphate to glucose releases -3.3 kcal/mol. on the other hand the hydrolysis of phosphoenolpyruvate to pyruvate releases -14.8 kcal/mol. As i said ATP is an intermediate energy molecule. This make sense since it is reactions such as phosphoenolpyruvate to pyruvate that can phosphorylate ADP back to ATP by substrate level phosphorylation. For such a reaction to occur it has to release more free energy than is required to make ATP (7.3 -12 kcal/mol) and of course it does. If ATP was a high energy molecule it would be much harder to synthesis it in the cell. David D. (Talk) 06:22, 7 February 2006 (UTC)
- Again, thanks DavidD - FrankH 05:42, 12 February 2006 (UTC)
- For what it's worth, the molecule that the professor pointed to in that episode and called "ATP" wasn't actually ATP; it was the supramolecular complex called "ATP synthase", whose role is to make ATP. I guess a small molecule just doesn't look as sexy on screen as a huge protein.
- I find this all to be very ambigious as it depends on many things. Like what are we counting as ATP? Are we including ADP and AMP? 5'3' cAMP? WikipedianProlific(Talk) 12:22, 22 October 2006 (UTC)
- Since this is a question of turnover, we are discussing ATP; how many ATP molecules are produced per unit of time. If one condsiders this value for any given day it is much greater than the total pool of ATP, ADP and AMP. This is still a very ambiguous discussion, however, since it is never possible to get an accurate value. There are way too many variables. David D. (Talk) 22:21, 22 October 2006 (UTC)
- I find this all to be very ambigious as it depends on many things. Like what are we counting as ATP? Are we including ADP and AMP? 5'3' cAMP? WikipedianProlific(Talk) 12:22, 22 October 2006 (UTC)
Stored??
Just a question- I'm not a biochem expert, but I'm taking an in-depth bio course and I was a bit confused by the statements "ATP is able to store and transport chemical energy within cells." and "ATP molecules are also used to store energy during the process of photosynthesis, as well as being the store of energy output from cellular respiration." I thought that energy in ATP could not be stored... Or does this just mean that it works as an energy carrier and not necessarily as a "store"? Matt White 02:04, 14 February 2006 (UTC)
- It all depends on th time frame. It does store energy in the sense that it is not lost as heat. But you're right that in the long term it does not store energy. David D. (Talk) 12:56, 14 February 2006 (UTC)
- The term "store" in this context is misleading, and I'm thinking of altering it. Fat and carbohydrates are both storage mediums; ATP, no. – ClockworkSoul 15:25, 14 February 2006 (UTC)
- I made some quick changes, but they still don't feel quite right to me. Unfortunately, I don't have the time to polish it, so that task will have to fall to somebody else for now. – ClockworkSoul 15:30, 14 February 2006 (UTC)
- Actually none of these molecules stores energy per se. Fats and carbohydrates produce most of their energy only when burned in oxygen, and even the breakdown of glucose to lactic acid produces energy only because it's hydrated lactate and the bonds in COOH are also more powerful and stable than anything in a sugar or alcohol. The problem with ATP is that generations of students have been told that there's something special about those phosphate bonds, that they give energy when broken. They don't. All chemical bonds take energy to break, or they wouldn't exist in the first place. Energy from ATP is obtained when the phosphate bonds are broken AND the products are hydrated. It's the hydration energy that drives the whole process. If you didn't have water and the proper concentrations, the thing would go nowhere. In that sense, water is sort of the "second fuel" which make this process go, in somewhat the same way that oxygen is the forgotten part of the energy that "exists" in a bit of food. Actually the energy isn't in the food per se, but rather can be extracted from the potential combination of food/fuel and oxygen. Same with ATP. SBHarris 23:16, 20 October 2006 (UTC)
the value of activation energy
Does anyone can tell me, From which paper or book did they cite the sentance : "The net change in energy of the decomposition of ATP into ADP and an inorganic phosphate is -12 kCal / mole in vivo, or inside of a living cell, and -7.3 kCal / mole in vitro, or in laboratory conditions. "
- I would imagine every biochem text book. The -7.3 is the free energy for the rection at standard conditions (by definition everything at 1M concentration at pH 7, 25˚C and 1 atm pressure). The -12 is at phsiological condition where the energy charge has about 5 fold more ATP than ADP, compared to 1:1 at standard conditions. David D. (Talk) 07:26, 16 February 2006 (UTC)
ATP redirect
Currently the acronym ATP is the ATP (disambiguation) page. Given this fact, why do we have the text:
- "ATP" redirects here. For other uses, see ATP (disambiguation).
At the top of the page? Shouldn't it read something along the lines of:
- The acronym "ATP" redirects to ATP (disambiguation).
Or are their plans ahead to have the "ATP" redirect to this page? David D. (Talk) 16:38, 9 June 2006 (UTC)
Bafilomycin redirect
Why does Bafilomycin (BAF) redirect to the ATP page when there is nothing about Bafilomycin on the page? Dr Aaron 06:24, 21 July 2006 (UTC)
Is the density correct..??
The density is listed as 67 g/cm3 = 67000kg/m3... On the density page it is stated that "The most dense naturally occurring substance on Earth is iridium, at about 22650 kg/m3". On of these statements must be wrong.... I don't know where to check it though.... Kjaergaard 18:55, 4 September 2006 (UTC)
- Fixed. A vandal added that figure a while ago; thanks for pointing it out. TenOfAllTrades(talk) 14:58, 9 September 2006 (UTC)
ATP in the human body
I don't really agree with the figure of 200-300 mole of ATP for daily need. It would mean 150kg of ATP and I heard in a biophysic course that it was about the third of that . Should somebody add a reference to back those figure, and gave some precision about this need (ie, is it the effective comsuption of all our cells are what we need to eat as energy but fail to convert in atp?) —The preceding unsigned comment was added by 82.224.181.243 (talk • contribs).
- There is a Nature article that cites 9 x 1020 molecules of ATP are metabolised each second at rest. I'll have to find the reference again but it is the one i used to get the figure at the top of this talk page. If true, that equates to 130 moles per day. That is not so different to the figure above, especially if that human is active. David D. (Talk) 21:55, 27 September 2006 (UTC)
Reorganisation
I've had a go at reorganising:
1 Chemical properties 1.1 Ionisation of ATP in biological systems 2 ATP Metabolism 2.1 Synthesis 2.1.1 Anaerobic resipiration 2.1.2 Anaerobic resipiration 2.1.3 ATP production by NDKs 2.1.4 ATP production during photosynthesis 2.1.5 ATP recycling 2.2 Energy release 3 ATP in cell signaling 4 ATP use in nanotechnology 5 Reference 6 See also 7 External links
This needs a lot of work still, but I think my reorganisation best highlights some of the things that need to be done. Those in bold in particular need more research. Dr Aaron 04:14, 3 October 2006 (UTC)
- Where does ATP as a regular of the activity of enzymes and other proteins fit in?--Peta 06:07, 3 October 2006 (UTC)
hmm.. there's a typo in aerobic respiration. i cant seem to find it in the actual wiki text though.
3d Structure of ATP
I was going to add high quality 3d images of ATP, in the QuteMol style, but i have found that there are various ATP 'shapes' around. for example at http://xray.bmc.uu.se/hicup/ATP/ there are two different pdb files, one experimental and one ideal, both of them different from the one currently featured. What should i use? Any suggestion of other PDB files of ATP? Eventually with H atoms?
-
experimental
-
ideal
-
current version
-
yet another version, Animated this time!
ALoopingIcon 15:43, 5 October 2006 (UTC)
- IMO, pretty much anything but the idealized version. It looks like 1xdn is just an "ordinary" structure, not some high-resolution study of ATP or anything, and I imagine the angles are somewhat flexible; you can probably use whatever ATP conformation gives the best view of its shape. I rather like the current one. (I'd also go hydrogenless for the space-filling view at least.) Opabinia regalis 05:22, 10 October 2006 (UTC)
- Ok, thx for the comment. I will put the first one. If someone can point me to the pdb file used for the last image i would re-make also that. ALoopingIcon 10:03, 10 October 2006 (UTC)
- Before adding the image i created also the animated version. What do you think? it is too distracting? ALoopingIcon 21:57, 11 October 2006 (UTC)
- I haven't gotten around to editing the actual article much, but - I like how the animation shows the 3d structure but would rather see the rotation slowed down a bit. Also, having it in the same rough orientation as the others (ie, vertically flipped 180) might be clearer. Opabinia regalis 07:50, 15 October 2006 (UTC)
- Before adding the image i created also the animated version. What do you think? it is too distracting? ALoopingIcon 21:57, 11 October 2006 (UTC)
- Ok, thx for the comment. I will put the first one. If someone can point me to the pdb file used for the last image i would re-make also that. ALoopingIcon 10:03, 10 October 2006 (UTC)
Krebs not Kreb
The man is Sir_Hans_Krebs. So it is the Krebs cycle. . And, according to the Chicago manual, the possessive is Krebs's, not Krebs' -- I've fixed themDGG 18:12, 5 October 2006 (UTC)
- Let me pull a Google: Did you mean: Hans Adolf Krebs? – ClockworkSoul 20:10, 5 October 2006 (UTC)
- oops. I forgot to go back here and check. thanksDGG 04:39, 6 October 2006 (UTC)
Question
Shouldn't another hydrogen be connected to the oxygen on the lefthand side of the diagram?76.21.2.201 20:14, 25 December 2006 (UTC)James
- No... at the pH inside cells - also called physiological pH - the hydrogens that are not shown in the diagram are generally not there. At lower pH levels there are more H+ ions present, making it more likely that those oxygens will be protonated. – ClockworkSoul 01:20, 26 December 2006 (UTC)
Passed GA
A nice written article, very well-referenced and well illustrated. I am not a molecular biologist, so I assumed that all formulas are correct. Perhaps one bit of story when ATP was discovered is good to add for a common reader interests. Nice job! — Indon (reply) — 15:15, 2 January 2007 (UTC)
DOPING?
Is ATP leagal for sportsmen??? —The preceding unsigned comment was added by 88.148.149.131 (talk) 20:32, 21 January 2007 (UTC).
- Yes, but I wouldn't expect it to have any significant effect on strength or endurance, which is probably why it isn't controlled. TimVickers 20:34, 21 January 2007 (UTC)
B-Class
This article clearly qualifies as a good B-Class, hence it Good Article. However, the WP:Chem wikiproject doesn't have a GA-class, therefore I administratively rated it as B. After improvement of the remaining suggestions in the comment, it can be commended on the WP:Chem peer review page for promotion to A-Class. That's how it's done in WP:Chem. Wim van Dorst (Talk) 22:43, 14 April 2007 (UTC).
Can I have some of that crack you're smoking?
Typically, a human will use up their body weight of ATP over the course of the day.
This is not possible unless a human typically consumes their body weight in food every day, and excretes their body weight in waste every day, which they don't. People typically consume 2-3% their body weight in food per day, regardless of obesity.
No nuclear fusion occurs in the human body, so it can't produce mass from nothing. Unless I'm missing something and ATP can be used more than once, which means it's false that it's "used up" in the manner described above, and it's also false that ATP can't be stored. That would be a form of storage.
It's not possible to metabolize more energy than you consume, unless you're operating on a debt diet, in which case after the course of about a week the body will begin to catabolize its own brain and the specimen is severely malnourished and in danger of death.
--76.224.78.226 04:25, 26 July 2007 (UTC)
- Read the entire paragraph again carefully, if you still don't understand, please outline here what it is you are having difficulty grasping. Tim Vickers 04:41, 26 July 2007 (UTC)
- ATP is recycled - not synthesized de novo. Does that help? Dr Aaron 07:03, 26 July 2007 (UTC)
Automatic addition of "class=GA"
A bot has added class=GA to the WikiProject banners on this page, as it's listed as a good article. If you see a mistake, please revert, and leave a note on the bot's talk page. Thanks, BOT Giggabot (talk) 04:35, 10 December 2007 (UTC)
citations needed: ATP as high energy molecule
For the two needed citations about ATP as high energy molecule, citation 5 cited in the sentence before can be used: Nicholls and Ferguson, Bioenergetics 3, 2002, ISBN: 0-12-518121-3, chapter 3. Pfrederix (talk) 09:56, 15 April 2008 (UTC)
- Yes, I had intended that to be a citation for all of this, but have added the additional tags. Tim Vickers (talk) 14:59, 15 April 2008 (UTC)
need to change the interwiki
the hebrew interwiki was changed to he:אדנוזין תלת-זרחתי
but the article is locked and I canwt change it Motyka (talk) 10:54, 14 May 2008 (UTC)
- Done. Tim Vickers (talk) 16:04, 14 May 2008 (UTC)
ATP is not high energy and is unstable?
In the article it says: [5] ATP is commonly referred to as a "high energy molecule"; however this is incorrect, as a mixture of ATP and ADP at equilibrium in water can do no useful work at all.[5] ATP does not contain "high-energy bonds", and any other unstable molecule would serve as a way of storing energy, if the cell maintained its concentration far from equilibrium.[5]
I dont really agree with this phrase. It tries to imply that ATP is not a "high energy molecule" because ATP and ADP in equilibrium do no useful work. But in fact any kind of molecule (be it high energy or not) in equilibrium with its products also does no useful work. To make any sort of statement about the high (or not energy) of ATP one would have to compare the energy release from that molecule to others. The given argument does not prove such a thing.
Also in the article it says: "ATP is an unstable molecule and tends to be hydrolysed in water"
I am also not completely sure about the way this statement is written, although it is true that ATP is somewhat unstable (it is important to understand that everything is relative) and tends to be hydrolysed in water, it is exactly its stability (perhaps compared with other similar energetic molecules) that define its utility as an energy token. If ATP would not be stable the cell would not be able to keep the ATP - ADP system far from equilibrium, because ATP would be very quickly hydrolysed into ADP. Being more stable, it is only transformed into ADP when used by kinases.
- The above was not signed. I'll try to answer. There is a lot of mythology about ATP from those unfamiliar with chemistry. All chemical bonds take energy to break, and those in ATP are no exception. If it weren't for water, ATP would be a reasonably stable molecule, and would provide a source of energy for nothing.
It isn't ATP which is an energy source, anymore than gasoline is an energy source. Rather, the energy is released when gasoline is reacted with oxygen, and some weak bonds are broken, and stronger bonds are made. Thus, gasoline and oxygen together act like a battery, but it takes BOTH.
ATP is the same way-- the energy is not "stored" in the ATP, but in the combination of ATP and water, which acts as the second reactant. Energetically, ATP like all phosphate polymers would like to hydrolyze down to plain phosphate, because this produces the maximal number of phosphate-water bonds of hydration (phosphate is quite soluble). So the enery is released only when ATP hydrolyzes, and the energy comes from weak bonds breaking in ATP, and stronger bonds forming from ADP and P being disolved in, and reacting with, water.
The concentration things are, by themselves, the same as for any chemical reaction which has a certain enthalpy and entropy associated with it. Even hydrogen and oxygen have some water in equilibrium with them at any given temp, but not much. The fact that the cell maintains all these concentrations far from equilibrium simply means that free energy is available from the reaction to do work. But there's nothing intrinsically different about this than with any chemical reaction which releases energy one direction, and absorbs it in the other (at the same concentration). SBHarris 01:40, 17 September 2008 (UTC)
- I am not a chemist (a physicist), but I am willing to bet that the above is wrong. The phosphate bond is considered high-energy because its disassociation has a RELATIVELY large amount of free energy to do work or produce heat. This bond is available to do work independent of hydrolysis. For example, assume you have one ATP in the entire universe. This bond can break with quantum mechanics alone spontaneously (it's just not energetically favored, and can take a VERY long time). When this does break, the amount of energy in this bond can do work (about 7kCal/mol) which is greater than other bonds. It has nothing to do with the water being there. Taking your other example: gasoline has more energy stored in it than a pool of water. Now it's stable enough that it doesn't spontaneously combust, but that doesn't mean that the energy isn't stored in the gasoline. You don't have to use oxygen, alkanes can react via halogenation or just put it in a calorimeter and reflux it for a year and youll get out the energy and loose the gasoline at the end of the reaction. For that matter, just look at the Wiki article on 'reflux', why would anyone reflux for days if it only had to do with the solvent and solute? Taking a more obvious example, just take some C4 and shake it (no solvent necessary) and you'll realize very quickly that the energy is stored in the bonds (or bond-level configuration) and has nothing to do with the solvent. Now it's true that it may be a bit incorrect to call it "in the bonds"--it has more to do with the fact that so many negative charges are packed in a tight space with ATP, but this is why they call it a high-energy PHOSPHATE bond to distinguish it from other covalent bonds, but something is definitely high-energy, and it doesn't have to do with the water.
- Even if what I wrote above is completely wrong, the author has to reconcile the fact that almost any text book labels this bond as a 'high-energy bond' without the qualification that is explained in the Wiki article. For example:
- "A phosphoanhydride bond or other HIGH-ENERGY bond is not intrinsically different from other covalent bonds, High-energy bonds simply release especially large amounts of energy when broken by addition of water. For instance, the Delta G for hydrolysis of a phosphoanhydride bond in ATP (~7.3 kcal/mol) is more than three times the Delta G for hydrolysis of the phosphoester bond (red) in glycerol 3-phophate (~2.2kcal/mol. A principal reason for this difference is that ATP and its hydrolysis products ADP and P are highly charged at neutral pH. During synthesis of ATP, a large input of energy is required to force the negative charged in ADP and P together. Conversely, much energy is released when ATP is hydrolyzed to ADP and P. In comparison, formation of the phophoester bond between an uncharged hydroxyl in glycerol and P requires less energy, and less energy is released when this bond is hydrolyzed."
- I caution anyone reading this that the explanation given in the Wiki text certainly don't mimic the explanation in almost any text (which can be referenced in google books.) --68.195.89.254 (talk) 03:07, 6 October 2009 (UTC)
- You're simply wrong that if there was one molecule of ATP in the universe, and no water, you could get free energy (or any sort of energy) from breaking one of its bonds. The definition of a chemical bond is that it is a negative energy state. The bond would not form if energy was not released in its formation. That's what MAKES a bond (see chemical bond). As for the textbook explanation above, you'll see it's basiclly identical to what was in the article.
A phosphoanhydride bond or other HIGH-ENERGY bond is not intrinsically different from other covalent bonds. High-energy bonds simply release especially large amounts of energy when broken by addition of water.
In other words, if "high energy" bonds are no different from other covalent bonds, there's no reason to use a new term for them, is there? The new bonds formed when ATP is hydrolyzed are stronger than old bonds within water and ATP. A stronger bond means it take more energy to break it, not that you get more energy from breaking it. You never get energy from breaking a bond per se. Thus, you get net energy by pulling ATP and and water apart (breaking these bonds) and making the new hydrolysis product (making new bonds both in the new products, and also in hydrating the products). Put an acid in water where you can get more hydrogen bonds and dissociation, and you get heat (try it with H2SO4). Yes, you'd probably get net energy simply making new -H and -OH hydrolysis products, without hydrating the products, as hydrolysis of most polymers to the the monomer usually gives energy (it's actually exothermic, meaning the enthalpy drives it as well as the entropy). But there again, it's new bond formation that drives energy-release. And even so, you wouldn't get as much energy as you get when ALSO calculating the energy of solvation (which is the standard way it's always done with ATP in biology). SBHarris 21:22, 24 October 2009 (UTC)
- You're simply wrong that if there was one molecule of ATP in the universe, and no water, you could get free energy (or any sort of energy) from breaking one of its bonds. The definition of a chemical bond is that it is a negative energy state. The bond would not form if energy was not released in its formation. That's what MAKES a bond (see chemical bond). As for the textbook explanation above, you'll see it's basiclly identical to what was in the article.
Nice article, some questions
I have no issues with the content except that in a few places ATP is used as a synonym for adenine (and meaning the adenine-containing nucleotide); ATP is not a nucleotide. I think this can be fixed most easily by moving content about adenine-containing molecules other than ATP to other articles (such as Adenine).
Reading about the total amount of ATP present in the human body, I am left wondering how much ATP+ADP+AMP is present! Given the rate of turnover of ATP, there should be far more ADP. Yes? --Una Smith (talk) 03:46, 25 October 2008 (UTC)
Requested move
- The following is a closed discussion of the proposal. Please do not modify it. Subsequent comments should be made in a new section on the talk page. No further edits should be made to this section.
The result of the proposal was do not move Anthony Appleyard (talk) 10:52, 14 December 2008 (UTC)
This page should be moved to ATP, because all other usages of ATP are only known to a specific group, while adenosine triphosphate is learned about in every high school biology class, and every high school requires students to take a biology class. This makes it the most well-known and the most notable use of ATP, so Adenosine triphosphate should be moved to ATP.
Furthermore, many biology students needing information about ATP will search for "ATP", and, when reaching the disambiguation page, won't know which link to click on, since many biology textbooks never state that ATP is short for "Adenosine triphosphate". --Oboeboy (talk) 15:22, 9 December 2008 (UTC)
- I see you redirected the disambiguation page to this article before asking here that this article be moved to ATP. Asking if people think something is a good idea after you have already done it is a sure way to annoy them. As to the move, I don't think it is at all probable that a student could miss the section at the top of the disambiguation page called "Chemistry/Biochemistry". This move is a solution to a problem that doesn't exist. Tim Vickers (talk) 16:49, 9 December 2008 (UTC)
- Oppose. Of the 55 articles now linking to ATP (see list), a large percentage do not intend Adenosine triphosphate. Also, the list of entries on ATP is already long, and likely to grow longer still. Finally, requesting user Oboeboy makes an argument concerning the education of (highschool) students; in that context, arguably, the greater educational value is in keeping ATP a disambiguation page, because it provides students the vocabulary-building task of matching "ATP" to "adenosine triphosphate". --Una Smith (talk) 17:34, 9 December 2008 (UTC)
- Oppose. Using an acronym as title is not a good idea. Please give an example for a standard biology text book that doesn't spell out adenosine triphosphate for ATP – perhaps it takes some dedicated "due diligence" searching for the long hand in such book, which any student with two hands and eyes will be capable of. Malljaja (talk) 18:06, 9 December 2008 (UTC)
- Support redirect, though it was never put it to a vote this point was discussed on the ATP talk page a while ago. I feel the same way now as I did then, ATP's primary usage is as adenosine triphosphate. Wikipedia is a useful teaching tool indeed, but surely that cannot be used as a validation for one practice or another because it is first and foremost an encyclopedia. Additionally, AMP and AFP as molecules have not reached the primary status in the lexicon that ATP most certainly has. My comparison for ATP would be something like the DNA page. Don't make the article titled ATP, but make it redirect here with the disambiguation page listed at the top for someone who was searching for something else.D-rew (talk) 18:23, 9 December 2008 (UTC)
- Needs more study Certainly the primary meaning of ATP in the sciences is to refer to the chemical, but if you Google it as a general term (which I recommend, because I didn't believe the results myself till I did), you'll find things aren't so simple. There's a reason why the ATP dab page is so large, and it's not just a long list of trivial and rare stuff. SBHarris 19:30, 9 December 2008 (UTC)
- Now that google is using searchwiki, has Wikipedia set up a new policy for the Google Search establishing prominence method?D-rew (talk) 19:54, 9 December 2008 (UTC)
- Number of results on Google:
- Adenosine triphosphate - 1,150,000
- Alberta Theatre Projects - 11,000
- sociated Talking Pictures - 2940
- BAe ATP - 19,600
- ATP Oil and Gas - 29,400
- Academic Talent Program - 1270
- Armenia Tree Project - 5860
- Association of Test Publishers - 7810
- Association of Tennis Professionals - 115,000
- Authority to Proceed - 577,000
- Airline Transport Pilot License - 10,600
- Assistive Technology Practitioner - 154,000
- Associate of Thames Polytechnic - 2
- AppleTalk Transaction Protocol - 9710
- Automated theorem proving - 69,600
- Automatic Train Protection - 19,900
- Acquisition, tracking and pointing - 5520
- Anti-Tachycardia Pacing - 12,400
- Asynchronous Transfer Program - 8 —Preceding unsigned comment added by 75.34.66.193 (talk) 21:26, 9 December 2008 (UTC)
- These hits other than Adenosine triphosphate add up to 1,051,620, hence Adenosine triphosphate exceeds any one other term, but barely exceeds the sum of other terms. To me, that proves Adenosine triphosphate does not qualify as the primary topic per the guideline WP:PRIMARYTOPIC. --Una Smith (talk) 23:04, 9 December 2008 (UTC)
- The primary topic guidelines specify that it must receive the majority, not a large majority. Since "adenosine triphosphate" receives 1,150,000 results compared to 1,050,000 results for everything else added together (meaning it has more than 100,000 results more than all other uses combined), which is unquestionably a majority.
- These hits other than Adenosine triphosphate add up to 1,051,620, hence Adenosine triphosphate exceeds any one other term, but barely exceeds the sum of other terms. To me, that proves Adenosine triphosphate does not qualify as the primary topic per the guideline WP:PRIMARYTOPIC. --Una Smith (talk) 23:04, 9 December 2008 (UTC)
- Oppose. Adenosine triphosphate may be ATP's primary meaning to biologists, but not to the general population. Anthony Appleyard (talk) 22:53, 9 December 2008 (UTC)
- Oppose – First, as an "encyclopedia of everything", it would be "unwiki" to replace a disambiguation page with a redirect, even if the new target is quite a bit more common than the other topics. Second, the very first entry, clearly indicated by a "Chemistry/Biochemistry" heading and clear textual description, is the link to "adenosine triphosphate". This change makes no sense. After all, if the students in question can't figure out what to click after that, then they won't likely gain much from the article itself. – ClockworkSoul 22:56, 9 December 2008 (UTC)
- Unwikilike even if one usage is extremely more common? By that reasoning, NFL should be a disambiguation page, too. —Preceding unsigned comment added by 75.34.66.193 (talk) 23:17, 9 December 2008 (UTC)
- Point well taken. I still don't think that "ATP" is specific enough to adenosine triphospahte to warrant the move. I think for this kind of thing an "order of magnitude" rule would be a good guideline, in which such a move could be considered appropriate if its number of Google hits is more than roughly one order of magnitude greater than the sum of the hit counts for all other subjects. – ClockworkSoul 18:15, 13 December 2008 (UTC)
- Oppose - when I think of ATP, I think of the Association of Tennis Professionals. Hence (to me anyway) Adenosine triphosphate does not fulfil the primary topic criteria. Also, as per comments above, I would be extra cautious about making an abbreviation a primary topic. — SteveRwanda (talk) 16:07, 11 December 2008 (UTC)
- 'Oppose ATP may mean one thing in biology, but not ubiquitous enough usage for a general purpose reference work. So also oppose redirecting ATP to the chemical. older ≠ wiser 22:01, 12 December 2008 (UTC)
- Oppose. And I thought ATP was about Tennis. In any case the goggle hits above do not show which of these might more commonly use ATP. Given that it is a 3 letter acronym I believe that you would need overwhelming evidence that there is a primary usage for one of these better known as the acronym to support this move. I simply don't see that being possible here. So ATP should be the dab page. I also find the nominators logic that we use what is being taught in high school rather weak and not compelling.Vegaswikian (talk) 23:53, 12 December 2008 (UTC)
Comments
What about google is using searchwiki? --Una Smith (talk) 20:58, 9 December 2008 (UTC)
- Though this is a wiki policy tangent, I'm just wondering whether Google results are a decent guideline for a word's use anymore, as it has been used in the past, since you can now tailor Google search results to strongly include preference in the google search algorithim. D-rew (talk) 21:11, 9 December 2008 (UTC)
- The above discussion is preserved as an archive of the proposal. Please do not modify it. Subsequent comments should be made in a new section on this talk page. No further edits should be made to this section.
Requested move 2
- The following is a closed discussion of the proposal. Please do not modify it. Subsequent comments should be made in a new section on the talk page. No further edits should be made to this section.
The result of the proposal was speedy close: the discussion is already closed. – ClockworkSoul 02:33, 16 December 2008 (UTC)
This page should be moved to ATP. Adenosine triphosphate is the most common usage of ATP, as shown by the fact that the definitions for ATP on both Dictionary.com and Merriam-Webster Online give "adenosine triphosphate" without even mentioning any of the other meanings. This, along with the fact that "adenosine triphosphate" alone gets 100,000 more results on Google than all other meanings combined, shows that "adenosine triphosphate" is the primary definition of ATP. --Oboeboy (talk) 16:01, 15 December 2008 (UTC)
- Oppose. As per reason given in previous request, and nothing new added here.Malljaja (talk) 18:38, 15 December 2008 (UTC)
- Oppose Proposers says ""Adenosine triphosphate" is the only definition for ATP given by several online dictionaries" unfortunately The Free Dictionary by FARLEX is not one of them.; it lists 149 other definitions of ATP. The question is not entirely one of whether "Adenosine triphosphate" is more likely to be searched for than any other meaning, although that is a sine qua non for being the default. In this case, it appears from the data above to have less than a statistically valid majority. There is also a question of the appropriateness of an initialism (an acronym) having a non-disambiguation page as a default, which would also weigh against this move. Lastly, anecdotally (WP:OR), I asked the first person who came by what "ATP" meant, and he said, without a pause, "All Terrain Pickup". --Bejnar (talk) 20:34, 15 December 2008 (UTC)
- Strong oppose the rename as well as this relisting as an abuse of process! There was nothing in the previous discussion that could be taken as a reason to support an additional discussion. In fact the consensus was rather clear. I will also note, that the reason for the nomination was disputed in the first discussion and was not rebutted there. Can an uninvolved admin close the now? Vegaswikian (talk) 20:51, 15 December 2008 (UTC)
- Oppose and Speedy Close This same move request just failed YESTERDAY. TJ Spyke 01:02, 16 December 2008 (UTC)
Comments - round 2
- The fact that adenosine triphosphate may be a commonly used name is not a factor in this discussion. It has nothing to do with the most common use of ATP. Given this, the nominator is basically offering no reason for this nomination. Vegaswikian (talk) 20:58, 15 December 2008 (UTC)
- The above discussion is preserved as an archive of the proposal. Please do not modify it. Subsequent comments should be made in a new section on this talk page. No further edits should be made to this section.
factoid: why keep
Leaders are required to indicate a subject’s importance.
From Lead_section#First_sentence_content: “The article should begin with a short declarative sentence, answering two questions for the nonspecialist reader: "What (or who) is the subject?" and "Why is this subject notable?"”
What makes a topic notable or not to a nonspecialist is obviously subjective. It might be felt that ATP's role as “a "molecular currency" of intracellular energy transfer” is sufficient. But I differ since this is a bit abstract for nonspecialists —other biologists, secondary school teachers, students, young people deciding whether to take up biochemistry or not. Fortunately, a nontechnical fact exists that explains in a very concrete and immediate way why ATP is so important: your own body turns over a mass of this molecule equivalent to your own body weight. Mentioning that only adds a few words but it really does make clear its notability. Indeed, I guess for many readers new to biochemistry, it will be the difference between having or not the curiosity to read further.
The editors for PNAS also would seem to agree that this fact is central to the notability of ATP. This is because this "factoid" is used in the most recent issue to start a commentary piece that deals with ATP. (It is this PNAS commentary that I referenced --reasonable given that the PNAS is a credible source --though ideally a citation should be to a paper that specific establishes the calculation).
For these reasons, I suggest it should be kept. --LittleHow (talk) 10:17, 31 December 2008 (UTC)
- I'm sorry I wasn't very clear in my edit summary, my fault entirely. This is indeed an interesting fact, but it was already in the article (see second paragraph of Adenosine_triphosphate#ATP_recycling). The PNAS source could be added as an additional citation to this section, particularly since it is free on-line access. However, it doesn't really belong in the lead. Tim Vickers (talk) 16:31, 31 December 2008 (UTC)
- I agree that the factoid does not belong in the lead. I worked it in somewhere else; now it is in two places and that needs to be fixed. --Una Smith (talk) 17:00, 31 December 2008 (UTC)
- Here are more comments for why it is important to be in the lead and not only in the text.
- ATP is one of the few pages that is going to get a lot of quick views from people that are not familiar with biochemistry. People and students, for example, will find it wikilinked on pages dealing with exercise. They are likely to read only the lead. It is important that they can read something with which they can immediately relate even if they find cannot grasp chemistry ideas. It is therefore particularly important that its lead should be written from the perspective of those who do not know any biochemistry, and for whom even simple chemistry terms are alien.
- The factoid is not merely interesting: it is deep. The key biological thing about APT is that though hidden away unseen in cells, all biology runs on it. No one can properly be said to be biologically literate unless they are aware that ATP exists and it has this role. The factoid puts in a nut shell this reality: here is something that is so much part of cellular biology that even though it makes up only a tiny fraction of your body its constant recycling means that its turnover equals the weight of your entire body. Even if people do not grasp anything else on the page, you will want them after leaving this page to remember that.
- The guidance on writing leads Provide an accessible overview says "The subject should be placed in a context with which many readers could be expected to be familiar". It is hard to do that for most biochemicals: but here an opportunity exists in a few words to do that for ATP. It should be exploited. I suggest the last sentence on ATP's discovery is made into a new paragraph (it is a break conceptually with the previous discussion about ATP's role in cell chemistry) and the factoid is put at its end where it would link naturally on from Lipmann's discovery of its role as the main energy-transfer molecule in the cell.--LittleHow (talk) 20:23, 31 December 2008 (UTC)
- Here are more comments for why it is important to be in the lead and not only in the text.
- I agree that the factoid does not belong in the lead. I worked it in somewhere else; now it is in two places and that needs to be fixed. --Una Smith (talk) 17:00, 31 December 2008 (UTC)
- I can certainly see that argument. What do you think of my attempt at rewriting the lead? Tim Vickers (talk) 20:39, 31 December 2008 (UTC)
I have a related question. The article states Living cells maintain the ratio of ATP to ADP at a point ten orders of magnitude from equilibrium, with ATP concentrations a thousandfold higher than the concentration of ADP. Does this mean that at equilibrium the ratio of ATP:ADP is 1:10000000 (1:10^7)? That point could be made more clearly, when this equilibrium is first discussed in the article. --Una Smith (talk) 00:25, 1 January 2009 (UTC)
2.5 vs 3 ATP per NADH
This article says that there are 3 ATP formed per NADH in oxidative phosphorylation. I know that's an estimate, but maybe it should be stated that the actual number is 2.5 according to most biochemistry texts and the fact that ATP synthase releases generates one ATP for every 4 protons and NADH leads to 10 protons in the electron transport change which adds up to 2.5 ATP. I'm not gonna edit it because it is cited, but maybe a more updated citation would be appropriate. 24.29.233.109 (talk) 03:15, 29 January 2009 (UTC)
- Rich PR (2003). "The molecular machinery of Keilin's respiratory chain". Biochem. Soc. Trans. 31 (Pt 6): 1095–105. PMID 14641005.
This article deals with the question in quite a lot of detail. Tim Vickers (talk) 03:38, 29 January 2009 (UTC)
Interesting article. It states that there are 10 c subunits in ATP synthase which would correlate to the 10 protons that NADH pumps. Combine that with 3 ATP per complete turn and you get a 10:3 ratio of proton to ATP and thus one NADH yields 3 ATP. I'm looking at the 2009 edition of Mark's Basic Medical Biochemistry and it states that there are 12 c subunits which would support the 2.5 ATP for every NADH theory. So this one may depend on who's doing the research. Additionally, the article you added mentions the energy required to bring in ADP and Pi into the Mitochondria and export ATP would put the ratio at around 2.3 ATP per NADH. Given the variability in information, I guess leaving it at 3 is best for simplicity sake. 140.220.1.66 (talk) 02:56, 30 January 2009 (UTC)
Free energy change of ATP hydrolysis to ADP
The net change in heat energy (enthalpy) at standard temperature and pressure of the decomposition of ATP into hydrated ADP and hydrated inorganic phosphate is −20.5 kJ/mol, with a change in free energy of 3.4 kJ/mol. The energy released by cleaving either a phosphate (Pi) or pyrophosphate (PPi) unit from ATP, with all reactants and products at their standard states of 1 M concentration, are:
- ATP + H2O → ADP(hydrated) + Pi(hydrated) + H+(hydrated) ΔG˚ = -30.54 kJ/mol (−7.3 kcal/mol)
- ATP + H2O → AMP(hydrated) + PPi(hydrated) + H+(hydrated) ΔG˚ = -45.6 kJ/mol (−10.9 kcal/mol)
Why are there 2 values for the free energy change of ATP hydrolysis to ADP: 3.4 kJ/mol and -30.54 kJ/mol? Temporal User (Talk) 06:47, 6 March 2009 (UTC)
ATP injections
ATP is popular as an injection for sport horses and other competition animals (dogs, birds), and this is a matter of considerable interest in the media this week due to the deaths of 21 horses injected with a solution containing ATP (see Biodyl). In Australia, an injection solution of ATP is available by mailorder.[1]. Could someone here please help to put this use of ATP in perspective? Eg, how much is 12.5mg of ATP, in moles? --Una Smith (talk) 15:40, 23 April 2009 (UTC)
- This makes no sense as ATP is the currency of energy within cells, not between cells. However much you put into the blood it isn't going to get to where it would be needed. I'd file this firmly under "pseudoscience" myself. Tim Vickers (talk) 19:01, 23 April 2009 (UTC)
- Of course it makes no sense. But it is marketed to owners and trainers of racing animals as an "energizer", and in the current flap over 21 dead polo ponies and Biodyl it has induced many reporters and chat room participants to write silly remarks about doping. --Una Smith (talk) 21:10, 23 April 2009 (UTC)
- I beg to differ with my limited knowledge on this topic: It's not quite pseudoscience. See: Effects of ATP infusion on glucose turnover and gluconeogenesis in patients with advanced non-small-cell lung cancer. (Pubmed Article) I'm curious to know if ATP directly injected to heart muscle by EMT's will prevent further muscle death despite myocardial ischemia. Oxygen and glucose are needed to make ATP, which makes the cell go around. Theoretically, giving ATP directly to the tissue (ATP diffuses through membranes) Avkrules (talk) 18:23, 1 March 2012 (UTC)
Protonated vs. deprotonated
I was staring at the posted ATP structure and the listed molecular weight for a few minutes trying to figure out why the molecular weight doesn't add up. It's because the listed molecular formula and molecular weight are for ATP in the acidic form (with all phosphates protonated, neutral charge), whereas the posted structure has these phosphate groups deprotonated, I guess to model it's appearance at neutral pH. I don't know how others would want to handle this - perhaps a note would suffice. 128.91.231.27 (talk) 15:23, 26 June 2009 (UTC)
Energy Content of an Isolated Molecule of ATP
I am going to edit the “Physical and Chemical Properties” section of this article. The interpretation taken by the previous author about the energy content of a molecule of ATP is not completely correct. Here are the paragraphs in question:
“ATP is commonly referred to as a "high energy molecule". Such characterization, however, is misleading. As with any chemical reaction that has reached equilibration, a mixture of ATP and ADP that has reached stable equilibrium in water will result in no further net hydrolysis of ATP.[9] A better analogy is that ATP and water is a mixture of potential reactants like fuel and oxidizer; both are required for energy release to take place.
Similarly, ATP does not contain "high-energy bonds". There is nothing special about the chemical bonds, including the phosphate bonds, in ATP. As with all chemical bonds, energy is required to break the bonds in ATP and energy is not released from breakage of these bonds. The bond-breaking in ATP requires initial energy input as does bond-breaking in all chemical reactions, but in the hydrolysis of ATP this input is more than re-paid with the energy of formation of "hydration" bonds between the products (ADP + phosphate) and water. In other words, the hydration process for the phosphate products results in a release of energy that exceeds the amount of energy for breaking the phosphate bonds in ATP, hence resulting in a gain in a net energy release from these two reaction steps.”
The notion that the triphosphate moiety of ATP does not contain high-energy bonds is incorrect. The bonds that constitute the anhydride linkages between the phosphates in ATP are in fact higher in energy than the resulting bonds of hydration. The analogy used in the first paragraph is technically accurate; even though the author has a similar misinterpretation about the chemical bonds in the fuel and the oxidizer (I presume he is referring to hydrocarbons and oxygen, which have bonds that are indeed higher in energy than the resulting bonds of carbon dioxide and water).
In the second paragraph, the author contradicts himself. He postulates that the breaking and reforming of bonds releases energy, but maintains that the bonds broken in the first place were not high-energy. This disobeys the law of conservation of energy. In order for energy to be released, that energy had to first exist somewhere that it could be released from. That energy resides in the anhydride linkages between adjacent phosphates. While it is true that the cleavage of any chemical bond requires energy, the author perhaps does not understand what is meant by the notion of “bond-breaking,” and also does not realize that this definition is not applicable to the hydrolysis of ATP, as the bond-breaking and bond-forming do not occur in two separate steps, as is suggested. The energy required to break a chemical bond is called the dissociation energy, and what this term refers to is the energy required to dissociate a bond to form two radical species. This does not occur in hydrolysis. Hydrolysis is an example of an SN2 reaction, which proceeds by a single-step (disregarding any protonation/deprotonation), polar mechanism (no radical intermediates). The hydration bonds are formed in the same step that the anhydride linkage is broken, and so the energy needed for the dissociation of the gamma phosphate is provided in the same step as the formation of the lower-energy hydration bonds. The difference in energy between the anhydride linkage and the bonds of hydration is equal to the maximum amount of energy that could theoretically be used to do work.
To comprehend this process, visualize dropping a rock. The rock before being dropped represents the anhydride linkages between adjacent phosphates in ATP. It is of high energy, and this energy can be released. The rock falling can be thought of as the single-step mechanism of hydrolysis which while proceeding results in a change of energy, just as the potential energy of the rock is converted to kinetic energy as it falls. The rock hitting the ground represents the reaction yielding energy in the formation of the new state. The rock releases its energy when hitting the ground, producing, among other things, sound. The sound that is heard from the rock hitting the ground is analogous to the energy released that is used to do work. Nowhere in this process did the rock need to have energy put into it in order to start falling (unless you consider lifting the rock in the first place, which, continuing with this analogy, would be equivalent to the formation of ATP from ADP and Pi, but not prepping ATP itself with energy to have more released). The energy released by the rock hitting the ground did not spontaneously come into existence, just as the energy released by the hydrolysis of ATP did not. The energy was contained in the bonds constituting the anhydride linkage. Keep in mind that all of that was just an analogy, and obvious inconsistencies can be demonstrated, but the idea between both processes is the same.
I am going to remove the two paragraphs that I have described to be erroneous and replace them with the following:
The energy content of an isolated molecule of ATP is a consequence of the anhydride bonds that connect adjacent phosphates. Anhydrides exhibit increased reactivity compared to their corresponding acids. This is because the bonds that constitute an anhydride moiety are less stable (hence higher in energy) than the bonds that can be formed from nucleophilic substitution. In the case of ATP, the bonds formed from hydrolysis, or the phosphorylation of a residue by ATP, are lower in energy than the phosphoanhydride bonds of ATP. Upon enzyme-mediated hydrolysis of ATP or phosphorylation by ATP, this energy can be harnessed by a living system to do useful work.
This change of the article is necessary because of the relevance of ATP to all of biochemistry. The current insufficiencies of this article to do not properly demonstrate the energy content of an isolated molecule of ATP, as is necessary in the understanding of biochemical processes which require ATP as an energy source. While the thermodynamic explanation (that which explains energy stored in systems far from equilibrium) is helpful, it is a macroscopic observation based upon microscopic phenomena, and so explaining the energy of ATP with thermodynamics does not actually answer the question, it only delays the explanation one step more. In reality, only a quantum mechanical explanation could fully suffice in describing the energy of ATP, but for the purposes of biochemistry, a chemical explanation is more than sufficient.
Dimethylformamide 19 October 2009 —Preceding undated comment added 21:06, 19 October 2009 (UTC).
Answer to above objection
There are two issues here. The first is what we mean when we say "high energy bond." It's very confusing unless defined carefully. A strong bond (such as in CO or N2) gives off lots of energy when formed, and thus also requires lots of energy to break (energy conservation). Do we then call a strong bond "high energy" or "low energy"? A matter of convention. The pyrophosphate bond in ATP is weaker than many (400 kJ/mole or less) and is less than the strength of the bond in H2O (493 kJ/mole). Are we going to call this pyrophosphate bond "high" or "low" energy?? So far as I can tell, the weaker the bond, the "higher energy" you want to call it! Would really, really weak bonds (van der Waals type) be "really, really high" energy in your scheme? You say anhydrides are "less stable," thus "higher in energy." But I can make bonds as unstable as you like. Example: helium atoms bond to each other very gently, which is what allows you to liquify it. That's a REALLY high energy bond, I suppose. :)
Look, you are focusing on the wrong process. There's no energy "in" any bond. They are negative energy states, like a rock in a hole. Energy is released not when old bonds are broken (this requires energy) but when new ones are made. Yes, this includes the new bonds of the hydrated product, but whether this happens in one or two steps is irrelevant. It's still the energy-release of the new bonds formed which drives the process. ATP is unstable to hydrolysis with water, and gasoline is unstable to oxidation with O2. So? That doesn't make the C-H bond in gasoline "high energy". The energy to be released isn't "in" the gasoline!
I calculate from the enthalpy of formations of the liquid reactants and products, see: http://physics-of-molecules.odessit.org/library/db/thermodata_2400.pdf that the enthalpy for H4P2O7 + H2O --> 2 H3PO4 is 6.2 kcal/mole. So yes, that's a large fraction of what is released under "standard conditions" of the 1 M concentration reaction in excess water. But this still emphasizes that the role of the water molecule reactant cannot be ignored. Also, the whole reaction here does not turn on simple differences in single bond strengths, but also resonance stabilization in phosphate monomers, stearic hindrance and charge repulsion in polyphosphates, and (in the case of free energy) also entropy considerations whenever you do a depolymerization. See http://www.dentistry.leeds.ac.uk/biochem/lectures/thermo/thermodynamics.htm
The large amount of free energy released has got nothing to do with the breaking of the phosphoanhydride bond attaching to the terminal phosphate group (breaking this bond uses energy). Some books call this bond a high energy bond but this is misleading.
Yes, it is misleading. My point. It's just wrong to see "high energy" IN any given bond. It's misleading and wrong headed. SBHarris 22:48, 24 October 2009 (UTC)
Rebuttal
I'm new to Wikipedia editing, so I apologize if this is the improper format.
I'm going to have to get into a lot of detail to explain this, and as a result it is going to be lengthy. I presume that your focus is on a more macroscopic scale (thermodynamics) relative to my microscopic scale (organic chemistry, quantum chemistry). Just know that all macroscopic phenomena observed are ultimately the result of microscopic interactions, so attempting to explain the ability of a cell to store energy in a system far from equilibrium is ultimately not a sufficient explanation. It's a logical fallacy of begging the question. Thermodynamics is more a mathematical model for the behavior of physical/chemical systems rather than theory.
Your first assertion is that the assignment of high or low energy to a given system is arbitrary. Your point is sound, but not relevant. Do not forget that many things in science are arbitrarily defined. An electron is arbitrarily said to have a negative charge, for example. I don't think referring to the bonds in ATP "high in energy," as they often are, and then building upon that is too devious. That is the established convention, just like the charge of an electron. Your description of N2 and CO as containing a stable, low-energy bond is therefore consistent with the standard I am using. So is the bond that connects two helium atoms in its liquid phase. This bond is so high in energy that it is very close to the energy at which it would "break," and so it cannot exist at ambient temperatures (if you're confused, wait for further explanation).
I think it's important at this point to address the issue you brought up of negative energy. That, too, is merely a conventional standard. I don't think you'll contest the idea that a system cannot have negative energy, unless you compare it to a system which you arbitrarily define to have zero energy. Contrary to what I might have seemed to imply, I think that this standard is quite useful, especially in explaining how unstable bonds are simultaneously high in energy, and vice versa.
Consider this image regarding the energy associated with a sigma bond between two hydrogen atoms with regard to internuclear distance: http://image.tutorvista.com/content/chemical-bonding/bond-formation-potential-energy.gif
The zero-point energy has been arbitrarily defined as the energy of the bond when the nuclei are separated by an infinite distance. I would like you to notice several things: first, that there is a discrete, though not explicitly quantified, lowest energy configuration possible for H2. To build upon that, bonds can be said to have a discrete energy, even if defined against an arbitrarily assigned zero point. Also note that since the zero point for any bond is defined by their interaction when separated by an infinite distance, any chemical bond has the same zero-point energy relative to any other chemical bond (the electromagnetic force, the force responsible for the existence of chemical bonds, is always zero between objects of finite charge separated by an infinite distance). With this established scale, we can now say for certain that some bonds are in fact higher in energy (closer to zero) than others. Central to all this is that chemical bonds do have energy "in" them. Perhaps "in" is not the best word, but that doesn't change the concept.
Irrelevant to this argument, but I think still worth mentioning, is that if a chemical bond absorbs a photon with an energy equal to the difference between the energy of that bond and the zero point, that bond will dissociate.
A graph with a similar curve can be made for any chemical bond, including a van der Waals interaction, which is, according this standard, very high in energy relative to covalent bonds, because the lowest energy point on that kind of graph would be much higher than the lowest energy point on a graph for a covalent bond. Useful energy cannot be derived from van der Waals interactions only because there is no way to extract this energy.
I think you do have a point that the water (or hydroxyl group, in the case of serine, threonine, and tyrosine) reactant should be given more attention. After all, if it were not for the reaction of hydrolysis (or phosphorylation), bonds that are lower in energy could not be formed, and energy could not be relinquished.
You made the same contradiction just now as you did when you authored the paragraphs that I replaced. (I presume that since you are defending the former interpretation that you are the author.) You can't on one hand say that you understand the conservation of energy and then not explain where the energy generated from the hydrolysis of ATP (or the combustion of gasoline) came from. It is here that I would like you to realize that though the energy contained in ATP can only be released by forming new bonds, the new bonds formed are indeed lower in energy than the original phosphoanhydride bonds of ATP.
If any part of this was unclear, I would be happy to explain it further. I certainly don't mean to insult you in any of this; I would just like an article so relevant to biochemistry to be as scientifically accurate as possible.
Dimethylformamide 25 October 2009 —Preceding undated comment added 02:18, 26 October 2009 (UTC).
- Just to curve this very abstract discussion back to the article, any changes in definition, be it for high vs low energy or weak vs strong bonds in the context of ATP will need proper sources, or else it'll be in violation of WP's no original research stipulation. If this sort of dissent about how to define a high or low energy bond exists in the literature, by all means include it in the article with the proper references. But this will need a sizable group of advocates on either side, not simply a small group that decided to go against convention, as this would give undue weight to this minority opinion. In any case, before making any gross changes, I would urge any editor to familiarize themselves with WP's sections on sources and neutral point of view. Thanks! Malljaja (talk) 13:52, 26 October 2009 (UTC)
Rebuttal to rebuttal
You're not insulting me. However I do have a degree in chemistry and thus I don't need it explained in such detail. I agree with the facts as you've stated them, but point out that your use of a term is not helpful. Yes, a bond may be "higher energy" than some OTHER bond. A rock at the bottom of a shallow hole is at a state of "higher energy" than if it fell into a deeper one (even though both rocks have negative energy, thus lower energy, with respect to sitting on level ground). But you can't extract the energy from a rock in a shallow hole, unless you can FIND a deeper hole. This is very similar to the situation in chemisty, where all bonds have negative energy, and it's not that useful to talk about "high energy bonds" or "low energy bonds," when we're really talking about differences between one and another. There, it's a descriptor for a relationship where both parties must be named, not one. The bond in any compound X is likely to be higher energy than the bond in some other compound Y, and yet at the same time, lower in energy than the bond in some other compound Z. Calling a bond "high energy" is like calling an object "big" (or "small"). Wow, that's a big rock! Well, "big" with regard to WHAT?? You can complain that chemical bonds, like rubber bands, have an average strength and a likely-range for that property. But this also a losing argument, because the P-O-P bonds in ATP are bonds of very average strength for covalent bonds. As in the C-H bonds of gasoline, their claim to fame is in the energy available when they react with some specific other thing and form new specific OTHER bonds.
And even there, the free energy abailable is a function of many other things than simple bond enthalpy (or energy), or even the strict differences in these between those, and those of products and reactants. The article should explain this. Once upon a time, it did.
As for the idea of solving this with a battle of refs: I did quote a source above at the end of my last message where some lecturer complains that it is misleading to label specific ATP bonds as "high energy." And yes, I'm aware than you can find dozens of pop science books, and even some biochem books (bowing to convention) which do this anyhow. Even though they'd never do it for the chemical bonds in fats and oils, here there is just as much-- perhaps more!-- reason for it. Do you see texts writing the bonds in fats with little tildes ~ to show they can be used as energy sources, indeed to make ATP? No. This is irrational, but it happens.
It wouldn't be the first time. Wikipedia depends on trying to sort through masses of science writing, some of which is better than others. It occasionally happens that the "more common" view is incorrect.
Example: The conventional wisdom and most references, will tell you that helium raises the pitch of the voice when you breathe it. And many refs give reasons WHY. They are all wrong, because the best expert opinion is that no pitch change occurs (you are fooled by a timbre change). Similarly, I can find a boatload of references to the idea that E=mc^2 means that mass can be CONVERTED to energy (after which the mass disappears). Wrong also (mass is always conserved) but you'll have to go to some very high level relativity books to get the straight dope. All the intermediate texts get it wrong. And so on. This is where Wikipedia needs expertise, not librarians. SBHarris 18:16, 26 October 2009 (UTC)
Response
The more this debate goes on, the more I think that we are really just saying the same thing in two different ways. The question on whether or not it is useful to describe things relative to some standard is an interesting one. In my edit, I didn't explicitly call the phosphoanhydride bonds "high in energy," but rather said that they were higher than the bonds of hydration or phosphorylation. I assume that this is why you've allowed this edit to survive. After thinking about it, I do think you have a point that it doesn't make sense to describe something as "big" or "high" since that can only be established relative to some predetermined standard. I was previously arguing while adhering to that standard. I still think that there is in fact energy associated with bonds, and I suspect that you do too, regardless of how you want to word it.
If you're still unsatisfied with the article as it stands, perhaps we could work together to write an even better one. Just as you would like bonds not to be described as high or low in energy (unless relative to another bond, I suppose), I would like the article not to ride strictly on thermodynamics. I definitely think that thermodynamics is incredibly useful, but only when the molecular-scale interactions responsible for thermodynamic descriptions are accounted for.
Dimethylformamide 21:11, 26 October 2009 (UTC)
- I don't think there is energy associated with bonds, if you mean the breaking of them. There is energy in making them, but you have to account for the new ones that are made, and the energy liberated is from THERE, not the previous ones that are broken. There is no energy "hiding" in an ATP molecule. The energy is released when it combines with water (or some other thing which is phosphorylated) and new bonds are formed with the water, or the other phosphorylated molecule. New bonds we don't see when looking at an ATP, just sitting there.
Now, we have another editor who seems to be quoting from some other biochem text which (if the quote is correct) doesn't understand chemistry, either. There is no such thing as a "high energy bond." It's an invention of biochemists (who even draw these bonds as though they had a spring in them),and evidently don't know enough chemistry. Imagine if I described the bonds in gasoline as being "high energy," as evidenced from the fact I got a lot of energy when I burn it! But it may be unfixable on Wikipedia, because this is such a wide misconception, even in biochem books. I can find cites in the other direction, but then we just have a your citation vs. my citation war. I actually have a degree in chemisty, and I'm not going to waste my time with this. Congratuations on screwing up truth, Wikipedia. The popular incorrect view wins again. SBHarris 20:12, 19 February 2010 (UTC)
- Thanks for your comments Sbharris. I agree that this issue is difficult to resolve and that text books can be a poor source of information—though one needs to acknowledge that their authors have considerable expertise in the topics that they write about. From your comment starting with, "I don't think...", I sense that you're practising a good deal of free association, so I would like to re-iterate that inserting personal views on a topic, however well intentioned, are much, much less valuable than those that reflect knowlegde from the literature, because ultimately there'll probably be as many opinions as there are editors, thus rendering an entry useless. So you will need to give your sources here, which should be no problem for you as you state that you have a degree in chemistry, a discipline with a long history of thorough scholarly research. I've made a start here, using a respectable Biochemistry textbook which promulgates a concept of the energetic contributions of ATP and the underlying reaction mechanisms that are widely accepted—you're welcome to chip in, but I'd urge you to forgo the temptation to deride other disciplines or opinions. Thanks. Malljaja (talk) 21:26, 19 February 2010 (UTC)
Response to response (textbooks)
Okay, I’ll give you opinions of textbook writers, at random, off my shelf. Here’s an old text called BIOCHEMISTRY by Voet and Voet (1990). Chapter 15 on Introduction to Metabolism has a section actually titled: B. Rationalizing the ‘Energy’ in ‘High-Energy’ Compounds. (!) It says that bonds whose hydrolysis proceeds with large negative Go', (customarily more negative than -25 kJ/mole)
<blockquote=Voet and Voet>are referred to as “high energy” bonds or “energy rich” bonds, and are frequently symbolized by the squiggle (~). Thus, ATP may be symbolized as AR-P~P~P.
It goes on to note that there’s not much difference between the squiggle P~P bonds and the non-squiggle R-P bond, and says:
<blockquote=Voets>In fact, none of these bonds have any usual properties so that the term “high-energy” bond is somewhat of a misnomer (in any case, it should not be confused with the term “bond energy,” which is defined as the energy required to break, not hydrolyze, a covalent bond). Why then, should the phosphoryl-transfer reactions of ATP be so exergonic? The answer comes from the comparison of the stabilities of the reactants and products of these reactions. .
The Voets go on to note that this cannot be understood except by looking at other reactants outside the ATP molecule. Much as the case with gasoline and oxygen. But they are indeed “rationalizing” (their word, not mine) the use of this squiggle, and a term which they themselves (not me) call “somewhat of a misnomer.”
Now, you may agree with the Voets that rationalizing misnomers in science is a great thing to do, but I personally do not agree with them. That is the extent of the personal opinion here. There is no great disagreement on the science, at least in this text, except that I can tell you that many a student has been confused by the squiggle, which means nothing by itself, and instead thinks of it as something special-- a spring in which energy is somehow stored. It isn’t. No more than energy is stored in gasoline-—a concept that would be more apparent if the other half of the fuel equation had to be carried around (as for example, the liquid oxygen half of the propellant in a rocket). The energy is NOT in the gasoline, but the system. It is certainly not in any sense hiding out in gasoline bonds! That there is energy in ATP or its squiggle bonds per se is a nice piece of self-promotion by Lipmann, but it led to years of texts less clear about the reality than Voet is.
Here’s something older: Lubert Stryer’s BIOCHEMISTRY, 3rd edition, 1988, which I keep for the nice colors of the structural formulas. And here’s Stryer apologia (Chapter 13, p. 318):
<blockquote=Stryer>ATP is often called a high energy compound and its phosphoanhydride bonds are referred to as high-energy bonds. There is nothing special about the bonds themselves. They are high-energy bonds in the sense that free energy is released when they are hydrolyzed, for the reasons given above [italics in the original]. Lipmann’s term “high-energy bond” and his symbol ~P (squiggle P) for a compound having a high phosphate group transfer potential are vivid, concise, and useful notations. In fact Lipmann’s squiggle did much to stimulate interest in bioenergetics..
Okay, that’s Stryer’s opinion: “Vivid, concise, and useful”. Though, of course, stuck in place of something that is nothing special in and of itself, as a rationalization for somewhat of a misnomer. If you see. But tell me why all this is not proper grist for derision? SBHarris 01:57, 20 February 2010 (UTC)
See what you've done
Somebody drew my attention to the article on energy (see the TALK page), and in the lede, I found it talking about high energy bonds in gasoline! Now, that wasn't me, trying to make a point. I had to fix it, but that's the kind of wrong thinking perpetuated by this biochemical language. Are you going to address it, or not? SBHarris 19:24, 22 February 2010 (UTC)
"Endergonic vs endothermic", and "exergonic vs exothermic"
In the section "metabolism, synthesis, and active transport", the article says "ATP is consumed in the cell by energy-requiring (endothermic) processes and can be generated by energy-releasing (exothermic) processes." I don't really understand much of this subject, but, as the point in this context is not specific to energy in the form of heat (as expressed by the "therm" morpheme in these terms), as there is more to metabolic energy than heat (which is more a form of "loss" of energy rather than the energy that is directly used in this context, albeit the heat generated isn't a pure waste or counter productive all the time), wouldn't it be more proper to speak of endergonic and exergonic processes, respectively? Apparently the "thermic" part does not imply that it's all about thermal energy anyway, but I guess that the words with "gonic" at least emphasize the non-thermal aspect. --Extremophile (talk) 18:34, 12 May 2010 (UTC)
Chembox contents
The structures shown for ATP are inconsistent, as 1 and 3 show the phosphate groups to be fully protonated, but 2 does not..
Adenosine triphosphate is present in biological systems as the phosphate and not the "phosphoric acid". The acidity constants, pKa, of the three primary phosphate protons are below 2; the final proton is situated at the terminal phosphate group of the triphosphate chain of ATP and has a pKa of about 6.5. In other words, at the physiological pH of about 7.6 ATP is overwhelmingly present as a tetravalent anion. This is correctly stated in the Ionization in biological systems section. For more details see H. Sigel and R. Griesser, Chem. Soc. Rev 34 (2005) 875-900 doi:10.1039/B505986K.
Since the article is entitled adenosine triphosphate, the strucres of the deprotonated triphosphate should be shown, not the protonated form. Petergans (talk) 10:43, 10 September 2011 (UTC)
Protonated ATP?
The top picture in the infobox, the line-bond black and white diagram one, is wrong! Upon careful inspection, you will see that it shows protonated ATP, not regular ATP. You can see this because the singly bonded oxygens on the phosphate groups have hydrogens on them, whereas in the other pictures, they do not. Plus, the title of the picture has "protoniert" in the name, which is German for "protonated". I think either a suitable substitute should be found, or it should be removed. 184.44.130.174 (talk) 20:17, 1 November 2011 (UTC)
Fate?
I couldn't find anything here about the fate of ATP metabolites. Could someone add? Well, maybe metabolites is the wrong word? ATP is not all conserved, the side-reactions eventually remove it (I assume). So, residence time and biological fate? 71.31.147.72 (talk) 16:55, 22 November 2011 (UTC)
- See the adenine section in purine metabolism, which should be the main article for a small catabolic section here. AMP is enzymatically dephosphorilated, deribosylated, deaminated to inosine, then hypoxanthine, xanthine, and finally is oxidized to uric acid, and you pee it off. SBHarris 19:25, 22 November 2011 (UTC)
Structure of ATP (The First figure)?
The Structure of the ATP Seems to be incomplete. It seems that the foll. link has a better representation http://www.trueorigin.org/atp.asp — Preceding unsigned comment added by 202.138.120.38 (talk) 05:17, 28 November 2011 (UTC)
May we please clarify that there is no energy stored in these "high energy bonds" per se?
They aren't like little springs. It takes energy to break them, as (by definition) it does for any chemical bond. Generations of students have gotten the wrong idea, and it would be nice to correct it. SBHarris 01:51, 7 November 2011 (UTC)
- SB - it is false that all chemical bonds require (additional) energy to be broken. Chemistry 101.
Only bonds with an activation energy require additional energy. In addition, are you claiming that the energy stored in springs is not chemical in nature?! Do I misunderstand you? Limiting our discussion to chemically relevant ideas: fuels are materials with high energy bonds (by definition). The separation of the bonds from the atoms/molecules in a material is pedagological rather than factual, I agree. If it serves to mostly inform rather than mislead, I would consider it "correct".71.31.147.72 (talk) 16:18, 22 November 2011 (UTC) [edit]I am really hoping that everybody in this "energy" discussion has taken a semester or two of thermodynamics (or learned the equivalent somehow else). Part of this discussion seems to be pointlessly spiraling around the FACT that in Chemistry all Thermodynamic energy is relative. That is why the relevant quantities are DELTA G or DELTA H. To argue that since the quantity is relative to a standard state we shouldn't be normatitve precisely reverses the (IMHO) correct argument. We should refer to the standard state(s) (which usually are implicit in non-peer reviewed literature) when discussing "high" or "low" certainly. Context is important. Some reactions (I'm guessing) are endothermic at 7 miles and 120° which are exothermic at physiological conditions. So what?71.31.147.72 (talk) 16:31, 22 November 2011 (UTC) [edit2] Sorry. Did I misunderstand that we are talking about the high energy C-H (etc.) bonds in an diatomic oxygen rich environment? Isn't it true that if the bond energy of P-O were "high" then it (ATP) would NOT be an effective "calalyst" in these biochemical cycles?? Most efficiency in any Thermodynamic cycle is infintesimal changes, right?71.31.147.72 (talk) 16:48, 22 November 2011 (UTC)
- All bonds require an activation energy to break. That's Chem 101. No bond stores energy and just breaks down in vacuum like radioactive decay, shooting component atoms in both directions! The definition of a chemical bond is something that releases binding energy in formation between two or more atoms. So yes, all bonds release energy when made, and they all require energy to break.
Yes, it takes energy to break C-H bonds, even in a high-oxygen environment. The only reason net energy is released when you burn a hydrocarbon, is that you get more energy from making new C=O bonds and O-H bonds, than it takes to break the C-H and the O=O bonds. There is no "energy" somehow residing in gasoline C-H bonds, and they aren't "high energy" in any sense. To split up a hydrocarbon to carbon and hydrogen requires energy-- you can look up the enthalpy of formation of hydrocarbons from carbon and hydrogen, if you like.
The "standard conditions" of thermodynamics simply take into account concentrations and other entropy-changing factors, which allow thermal energy to be changed to chemical energy (and sometimes even vice versa, if the entropy term wins out and allows it). However, none of these properties are IN THE BOND. Because these processes happen in statistical systems with a temperature (a statistical property) and involve heat (which makes no sense except in the context of temperature) these things are all system functions. Here we talk about "free energy" but free energy is not "in" a bond, it's in a system. So writing those bonds in ATP with a different symbol just confuses things. That's not where energy is. It's not even where free energy is.
And finally, the energy stored in springs I think is rather more like the energy stored in compressed gases. No, it's not "chemical" in nature. It doesn't rely on chemical reactions. Do you think the energy stored in compressed helium is released by a chemical reaction when it expands? Well, energy you get from a contracting rubber band isn't any more "chemical" than that. (In metal springs, there may be some genuine electromagnetic potential energy storage involved, but in elastomers, there is not; they all absorb about as much heat as they do work). SBHarris 19:37, 22 November 2011 (UTC)
- So I hope that people don't mind if I jump in to this discussion and provide my two cents. As all parties have mentioned, this is really a case of deciding on a reference point for the energy. While I would agree that phosphate and all other involved reactants are higher in energy than the products of the reaction, I do not believe that this can be generalized into the statement that there is a "high-energy bond." The problem with "high-energy bond" is that the word "bond" inherently describes the reference state for energy. By long-held convention, the energy of a bond is defined with respect to the potential energy of the two bond fragments when separated by an infinite distance. In agreement with this convention, any state of the "bond" in question that has a energy level higher than this reference energy is, by definition, not bound. In fact, the long-held convention arises due to this reason. Any interaction with an energy higher than the potential energy of the infinitely-seperated fragments cannot be classified as a bond. At best, it could be described as a glancing interaction between two weakly-interacting particles. This is similar to inter-molecular potentials in the gas phase. Just because two molecules have some measureable interaction between one another (perhaps by dispersion forces or a dipole-dipole interaction) in the gas-phase, does not mean that they are "bonded" to one another. They simply have too much energy to be considered as bonded to one another.
- All bonds require an activation energy to break. That's Chem 101. No bond stores energy and just breaks down in vacuum like radioactive decay, shooting component atoms in both directions! The definition of a chemical bond is something that releases binding energy in formation between two or more atoms. So yes, all bonds release energy when made, and they all require energy to break.
- So how does this all relate to the case of phosphate? I would agree that the reactants can and should be classified as a "high-energy state" since their collective potential energy is higher than the products. In this case, the reference for the energy is the minimum potential of the collectively-more-stable products. However, classifying the individual "bond" as "high-energy" is simply wrong because the stability of a bond is inherently determiend by the separation of the fragments at infinite distance. The bond could simply care less what the energy is for another molecule. It has no knowledge of any other reference state. Sirsparksalot (talk) 17:50, 8 December 2012 (UTC)
Misleading idea that breaking bonds releases energy
I am thinking of breaking [....] [High-energy phosphate]] up into sections so that the final paragraph on the misleading use of the term 'high-energy' is emphasized more. As this paragraph states, bond breaking almost always requires input of energy. The energy released is due to the net total hydrolysis reaction, not the 'bond-breaking' process. I would like to do this because I cannot count the number of students I have had whom were taught in biology that "breaking bonds releases energy." Does anyone have a problem with this approach? Sirsparksalot (talk) 14:42, 12 November 2012 (UTC)
- Not me!! I've had major trouble even getting this point into the adenosine triphosphate article to the extent that it's there now (see the TALK section). Bond breaking ALWAYS requires energy, or it wouldn't be a bond. The opposite idea is one of the most pervasive falsehoods in science, akin to the idea that mass can disappear and be converted to energy (that's matter not mass). I blame Lippman's 1941 squiggle bond representation, that looks like a coiled spring. A nice bit of showmanship for his ideas, but a source of endless misconception as a result, for 70+ years. SBHarris 19:30, 12 November 2012 (UTC)
- I would check out the talk page but I can't seem to find the archives. In any event, I can certainly understand your frustration and am sorry that I wasn't here at the time to help your cause! As for my original comment, I was not thinking of providing an overhaul of the article, just creating a new subsection with the heading "Miconceptions about bond-breaking", or something to that effect, and using the paragraph presently at the end of the article. If you think it is best left as is then I won't change it. Maybe it's something we can keep in mind for the long term? On another note, I was hesitant to say that bond breaking always requires energy because there are some (albeit very few) well-documented examples of metastable bonds that will release energy when broken. However, such examples are exceedingly rare and only occur in very special circumstances. Suffice it to say that they are virtually non-existent in naturally-occuring phenomena, including phosphate bonds. However, the scientist in me refrains from using the word "always." Sirsparksalot (talk) 23:22, 25 November 2012 (UTC)
- There are no archives; all this is in the regular ATP TALK page. Just start here and read downward. Horrifying. Do feel free to make changes and back me up. My problem was in being one guy in sea of people who had read sloppy texts. I have had a few other experiences like it on WP: at the articles on weight and heat for example. But if there are two of us, one can take on the Injuns while the other sleeps!
Didn't even realize there were metastable chem bonds. One bond? I see no reason it can't happen in theory, but these things happen in timescales usually of 10^-8 sec, so metastable would mean "only" 10^-3 sec? In metastable nuclear isomers you can get kinetic stabilty from high spin forbiddenness (ala Ta-180m). BUt there's not much to hang you up in a single chemical bond. Electrons in a single bond soon do what they want. It takes big collections of atoms (preferrably solid) to stop them. But in those cases the lower energy state is a very complicated distance away from the higher energy state, and single bonds aren't involved. Do you have a best example? SBHarris 01:21, 26 November 2012 (UTC)
- Fun times! I now see why you are so hesitant to make edits. There is certainly a lot of confusion and mis-information out there. I think that the root of the problem is that chemistry is very rarely taught "one molecule at a time," but instead as "one reaction at a time." In principle, this isn't a flawed approach since single-molecule reactions are exceedingly rare in nature. However, people seem to forget that anything that happens to one compound is inherently coupled to another molecule. That being said, it did take me a lot of time to figure this out. In retrospect, if I didn't have a background as a gas-phase physical chemist, I'm not sure if I would have been exposed to the type of information to to figure it out at all.
- There are no archives; all this is in the regular ATP TALK page. Just start here and read downward. Horrifying. Do feel free to make changes and back me up. My problem was in being one guy in sea of people who had read sloppy texts. I have had a few other experiences like it on WP: at the articles on weight and heat for example. But if there are two of us, one can take on the Injuns while the other sleeps!
- I would check out the talk page but I can't seem to find the archives. In any event, I can certainly understand your frustration and am sorry that I wasn't here at the time to help your cause! As for my original comment, I was not thinking of providing an overhaul of the article, just creating a new subsection with the heading "Miconceptions about bond-breaking", or something to that effect, and using the paragraph presently at the end of the article. If you think it is best left as is then I won't change it. Maybe it's something we can keep in mind for the long term? On another note, I was hesitant to say that bond breaking always requires energy because there are some (albeit very few) well-documented examples of metastable bonds that will release energy when broken. However, such examples are exceedingly rare and only occur in very special circumstances. Suffice it to say that they are virtually non-existent in naturally-occuring phenomena, including phosphate bonds. However, the scientist in me refrains from using the word "always." Sirsparksalot (talk) 23:22, 25 November 2012 (UTC)
- As for your question about metastable chemical bonds, the are quite rare and are generally isolated to multiply-charged ions in the gas-phase. Some have lifetimes of 10^-3 sec (as you predicted) and I believe that some have been stored for as long as seconds. I can try to dig up some references if you would like. Because of the excess charge on the ion, there is long range repulsion involved in forming the bond. From long distances, one charged fragment is repelled from another like-charged fragment. At short distances, this repulsion is masked by what can be thought of as "normal" covalent bonding, which arises from the interaction of fragment electrons with the nuclear framework of the other fragment. While I know only very little about nuclear physics, I will go out on a limb and say that the bond metastability is similar in nature to the metastable nature of nuclei, which arises from the long-range Coulomb repulsion of nucleons combined with the short-range strong/weak nuclear attraction. For chemical bonds,the timescale is dictaed by entropic effects and tunnelling. Typical molecules have some degree of residual energy in them and unimolecular decay will be dictated by the amount of time necessary for energy to find its way through phase space into the right nuclear coordinate. Typically, molecular fragments are too large for tunelling to occur on a reasonable time scale, unless the molecule has sufficiently-high energy to be close to the top of the barrier. All this being said, once you add solvent to the mix, it is able to polarize and reduce the effects of the long-range electrostatic repulsion between the moleucle framents. This makes the molecule go back to a regime of normal, stable bonding (much like we would think of an H2 potential energy surface).
- Unfortunately, all of this lends some confusion for the case of phosphate bonds. I will have to go back, dig-up, and review some references to be sure, but I'm almost 100% certain that phsphate, because of its highly-negatively-charged state, won't form a chemical bond in the gas phase. From what I can gather, a lot of people use this general idea of phosphate's Coulombic instability to justify the "breaking a bond releases energy" argument. What people fail to realize is that this is only true in the GAS PHASE. In fact, if they really thought about it, this wouldn't even help their case since, for phosphate in the gas-phase, there is no bond to be broken! The only reason that phosphate as we know it exists is is because of solvent screening effects!
- Anyway, all that aside, phosphate bond breaking does not occur in a vacuum but in aqueous solution. This means that, in addition to all the quantum effects present in all of the bonds of all involved species, the differential solvation of all reactants and products MUST be included. In these cases, we have to treat it just like any other stable bond (more or less), e.g. H2. In other words, breaking a bond, even phosphate, requires energy. If it wasn't coupled to a hydrolysis reaction, it would be an endothermic process and life, so far as we know it, wouldn't exist.
- Okay, I'll stop preaching now. Hopefully that answered your question about metastable bonds. I'll see if I can help out on the other talk page when I get some more time to find a good entry into the thread. If you think any of the above conversation helps your cause, let me know and I can paste some of it into other talk pages. Sirsparksalot (talk) 17:12, 8 December 2012 (UTC)
I think I'll paste this entire section (from the TALK page of high-energy phosphate) into the adenosine triphosphate TALK page at the appropriate place (and here you are, folks!), complete with header. It never hurts for doctors and biochemists to learn more basic chem. Here's a great example of the foolishness that happens when they get it second or third hand. I do not except myself from such errors. SBHarris 20:46, 8 December 2012 (UTC)
Energy? What Kind of Energy?
At first I thought it was caloric energy, since it seems to be measured in calories!? If so, then it must work in the manner of quick lime... Next I quickly discounted mechanical energy, and god forbid, it surely cannot be electrical energy.
Other than that, I cannot think of anything else, but strange mather or exotic partcles, so, please edify me by replying, or better yet editing the article and making the topic of ATP "energy" more clear. 67.206.184.169 (talk) 06:10, 7 June 2013 (UTC)
ATP Recycling Error {PLEASE FIX}
In ATP recyling, at the start, it says that there are .2 moles of ATP at any given time, and the molar mass of ATP is 507.18 g mol, so approximately 100 grams at any time. However, in the end of the section of ATP recycling, it says that there is only 5 grams of ATP at any time. I'm not a chemistry professor or anything so I don't feel comfortable changing it. Just a random anonymous user pointing out that something doesn't add up. Or maybe it's just me. — Preceding unsigned Edit-Also, in the second paragraph of the introduction at the very front of the article, it states that there are 250 grams of ATP in the body at any time. So we have three numbers that all seem to be for the same thing, 5, 100, and 250 grams.
Chemistry Beginners
Perhaps you could make a simplified definition of ATP, how it is made, and what its' function is, etc. — Preceding unsigned comment added by 75.76.24.37 (talk) 19:06, 14 December 2013 (UTC)