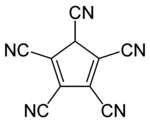
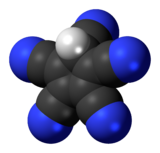
Pentacyanocyclopentadiene
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclopenta-1,3-diene-1,2,3,4,5-pentacarbonitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10HN5 | |
| Molar mass | 191.153 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pentacyanocyclopentadiene is a derivative of cyclopentadiene with five cyano groups with the molecular formula C5H(CN)5. The corresponding anion, pentacyanocyclopentadienide, is a ligand with the molecular formula C
5(CN)−
5. In contrast to other anions based on a C5 ring unit it binds to metals through the pendant cyano groups rather than the C5 ring. The anion was first synthesised by Webster in the 1960s[1] and its conjugate acid much later on.[2] More recently Wright has discovered its extensive coordination chemistry.[3][4] By virtue of a combination of mesomeric and aromatic stabilization of its anion, pentacyanocyclopentadiene is a superacid, with an estimated aqueous pKa of −11.[5] The free acid was prepared by Reed in 2004 and was assigned a polymeric structure with protons that bridge planar C5(CN)5 units.[6]
Synthesis[edit]
Pentacyanocyclopentadiene is synthesised by coupling carbon disulfide and sodium cyanide in dimethylformamide before oxidation using ammonium persulfate and final purification generates the ammonium pentacyanocyclopentadiene salt. Further reaction with sodium hydride generates NaC5(CN)5 which is a starting point for its coordination chemistry with transition metals.
Reactions and coordination chemistry[edit]
Coupling of sodium pentacyanocyclopentadiene (NaC5(CN)5) with transition metal halide salts generates metal complexes containing the C
5(CN)−
5 anion.[3][4]
Because the anion binds to metals through the cyanide group it can act as a pentagonal node. Thus it can form fullerene-like structures with large voids containing solvent.[7][8] This has important implications for gas storage and separation.
References[edit]
- ^ Webster, O. W. (1966). "Diazotetracyanocyclopentadiene". Journal of the American Chemical Society. 88 (17): 4055–4060. doi:10.1021/ja00969a029.
- ^ Reed, C. (2004). "Exploration of the pentacyano-cyclo-pentadienide ion, C
5(CN)−
5, as a weakly coordinating anion and potential superacid conjugate base" (PDF). Chem. Commun.: 706. doi:10.1039/b316122f. - ^ a b Wright, D. S. (2011). "Transition metal complexes of the pentacyanocyclopentadienide anion". Chem. Commun. 47 (36): 10007. doi:10.1039/c1cc13021h. PMID 21833428.
- ^ a b Wright, D. S. (2012). "Group 11 complexes containing the [C5(CN)5]− ligand; 'coordination-analogues' of molecular organometallic systems". Dalton Trans. 41 (19): 5919. doi:10.1039/c2dt30274h. PMID 22473357.
- ^ Jonathan, Clayden; Greeves, Nick; Warren, Stuart G. (2012). Organic Chemistry (2nd ed.). Oxford: Oxford University Press. ISBN 9780199270293. OCLC 761379371.
- ^ Richardson, Christopher; Reed, Christopher A. (2004). "Exploration of the pentacyano-cyclo-pentadienide ion, C
5(CN)−
5, as a weakly coordinating anion and potential superacid conjugate base. Silylation and protonation". Chem. Commun. (6): 706–707. doi:10.1039/B316122F. ISSN 1359-7345. - ^ Bacsa, J. (2011). "Assembly of the First Fullerene-Type Metal-Organic Frameworks Using a Planar Five-Fold Coordination Node". Angew. Chem. Int. Ed. 50 (36): 8279–8282. doi:10.1002/anie.201102783.
- ^ Less, R. J. (2013). "Solvent Direction of Molecular Architectures in Group 1 Metal Pentacyanocyclopentadienides". Eur. J. Inorg. Chem. 2013 (7): 1161–1169. doi:10.1002/ejic.201201342.
