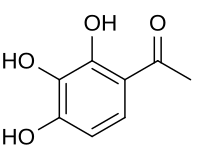
Gallacetophenone
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(2,3,4-Trihydroxyphenyl)ethan-1-one | |
| Other names
1-(2,3,4-Trihydroxyphenyl)ethanone
Alizarin Yellow C Galloacetophenone 2',3',4'-Trihydroxyacetophenone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.665 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.148 g·mol−1 |
| Melting point | 171 to 172 °C (340 to 342 °F; 444 to 445 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Gallacetophenone is the acetyl derivative of pyrogallol. It can be synthesized from pyrogallol using zinc chloride and acetic anhydride.[1]
References[edit]
- ^ talkchem.com https://web.archive.org/web/20100116013814/http://talkchem.com/synthetic-chemistry/gallacetophenone-synthesis-synthesis-of-gallacetophenone.html. Archived from the original on January 16, 2010.
{{cite web}}: Missing or empty|title=(help)
